Best Disease Gene Therapy Advances Toward Clinical Trial
Eye On the Cure Research News
Using gene therapy, FFB-funded researchers at the University of Pennsylvania School of Veterinary Medicine (Penn Vet) and Perelman School of Medicine have reversed the disease process in a canine model of Best disease, an inherited form of macular degeneration that can lead to severe vision loss in humans
Audio version:
Using gene therapy, FFB-funded researchers at the University of Pennsylvania School of Veterinary Medicine (Penn Vet) and Perelman School of Medicine have reversed the disease process in a canine model of Best disease, an inherited form of macular degeneration that can lead to severe vision loss in humans. The therapeutic effect of the treatment has been sustained for as long as five years. Results of the study led by Karina Guziewicz, PhD, and Artur Cideciyan, PhD, were published online in the journal Proceedings of the National Academy of Sciences (PNAS).
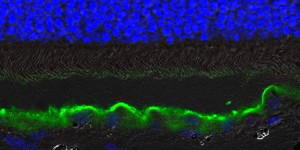
Untreated retina
"With this research," Guziewicz says, "we have demonstrated that gene therapy is working in a large animal model. Following safety studies, a human clinical trial could be two years away."
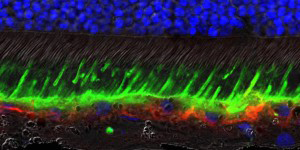
REtina treated with gene therapy
While the Penn researchers have been studying Best disease in canines and humans for several years, they recently found that a microscopic separation between photoreceptor cells and retinal pigment epithelium (RPE) is the primary retina-wide abnormality. This eventually leads to vision-threatening lesions, degeneration, and vision loss.
Photoreceptors are the light-sensing cells that make vision possible. The RPE is a single layer of cells that provides a number of support functions for photoreceptors including delivery of nutrients and waste management. In the normal retina, photoreceptors are nestled in the RPE sheet.
Lesions comprised of waste material accumulate in the RPE and within subretinal space of humans and dogs with Best disease. The research team observed that the gene therapy in dogs eliminated the lesions as well as the microscopic separation between the photoreceptors and RPE.
The research team also evaluated patients with Best disease to better understand the vision loss associated with the separation between photoreceptors and the RPE. "The flow of nutrients between RPE and photoreceptors normally occurs over a very small distance," Cideciyan says. "So, if you have a separation between these two layers, flow of nutrients takes longer. This causes a substantial slowing of the recovery of vision following exposure to bright light. The implication is that, if we could correct the separation, we would also correct the visual defect."
"Penn Vet's gene therapy research for Best disease has been elegant and groundbreaking," says Stephen Rose, PhD, chief scientific officer at FFB. "Their outstanding work positions the treatment well for translation into humans."
FFB has been funding Dr. Guziewicz for her Best disease research in canines since 2008.
In addition to Drs. Karina Guziewicz, and Artur Cideciyan, the research team included Penn Vet's Gustavo Aguirre, VMD, PhD, William Beltran, VMD, PhD, AndrÌÁs M. KomÌÁromy, DVM, PhD, ValÌ©rie L. Dufour, DVM, Simone Iwabe, DVM, PhD, Gordon Ruthel, PhD, and Brian T. Kendrick; Penn Medicine's Samuel Jacobson, MD, PhD, Malgorzata Swider, PhD, and Alexander Sumaroka, PhD; the University of Florida's Vince A. Chiodo and William W. Hauswirth, PhD; and the University of Toronto's Elise HÌ©on, MD.




