
Often diagnosed in childhood or adolescence, Stargardt disease is an inherited form of macular degeneration causing central vision loss. The condition is sometimes referred to as juvenile or early onset macular degeneration.
What is Stargardt Disease?
Stargardt disease is a genetic eye disorder that causes retinal degeneration and vision loss. Stargardt Disease is the most common form of inherited macular degeneration, affecting about 30,000 people in the U.S. The progressive vision loss associated with Stargardt disease is caused by the degeneration of photoreceptor cells in the central portion of the retina called the macula.
The retina is the delicate light-sensing tissue lining the inside wall of the back of the eye. Photoreceptor cells in the retina convert light into electrical signals, which are sent to the brain where they are processed to create the images we see. The macula, which is rich in cone photoreceptors, is responsible for sharp central vision — for tasks like reading, watching television, and looking at faces. Cones also provide vision in lighted settings and color perception.
Decreased central vision due to loss of photoreceptors in the macula is the hallmark of Stargardt disease. Some peripheral vision is usually preserved. Stargardt disease typically develops during childhood or adolescence, but the age of onset and rate of progression can vary. The retinal pigment epithelium (RPE), a layer of cells supporting photoreceptors, is also affected in people with Stargardt disease.
Symptoms
A loss or change in central vision is what usually leads to the initial diagnosis of Stargardt disease. A retinal doctor examining the retinas of a person with Stargardt disease will see characteristic yellowish flecks in the RPE. The flecks are deposits of lipofuscin, a byproduct of normal retinal cell activity. However, in Stargardt disease, lipofuscin accumulates abnormally.
The progression of vision loss in Stargardt disease is variable. Visual acuity (the ability to distinguish details and shape) may decrease slowly at first, accelerate, and then level off. Some peripheral vision is usually maintained.
Inheritance
Stargardt disease is usually an autosomal recessive condition caused by mutations in the gene ABCA4. It is inherited when both parents, called carriers, have one mutated copy of ABCA4 and a normal copy. Each child has a 25 percent chance of inheriting the two copies of ABCA4 (one from each parent) needed to cause the disease. Parents are unaffected carriers because they have only one mutated copy of ABCA4.
In a small percentage of cases, Stargardt disease is caused by mutations in the gene ELOVL4 and is passed on to children through the autosomal dominant inheritance pattern. In these cases, the parent has the disease and has a 50 percent chance of passing the mutated copy of ELOVL4 (and the disease) on to each child.
Living with the Disease
There are many services and accommodative and assistive resources available to people and families with Stargardt disease. Visit the Foundation’s Low Vision Resources page to learn about many of these resources. A low vision specialist can help recommend the resources that are right for you.
More information on managing Stargardt disease is in the Newly Diagnosed section of this website.
Genetic Testing
Genetic testing is available to help people definitively diagnose their condition and the risk of other family members or future offspring being affected. A genetic diagnosis can also help a person qualify for a clinical trial. Genetic counselors are excellent resources for discussing inheritability, family planning, genetic testing, and other related issues.
Research and Clinical Trials
For the latest research advances for Stargardt disease treatments, refer to the Foundation publication Stargardt Disease: Research Advances.
View a list of current clinical trials, many made possible by Foundation support
Stargardt disease page at www.ClinicalTrials.gov.
Next Section
Latest News
-
.png)
Jan 20, 2023
Eye on the Cure Podcast – Episode 39: Paul Bernstein, MD, PhD
Foundation PodcastsJanuary 20, 2023. Paul Bernstein, MD, PhD, from the Moran Eye Center, University of Utah, and a member of the Foundation’s Scientific Advisory Board talks to host Ben Shaberman about his clinical practice for retinal disease patients
-
.png)
Dec 16, 2022
Eye on the Cure Podcast – Episode 37: Rando Allikmets, PhD
Foundation PodcastsDecember 16, 2022. Rando Allikmets, PhD, talks to host Ben Shaberman.
-

Nov 9, 2020
Stargazer Pharmaceuticals Initiates a Phase 2a Clinical Trial for its Stargardt Disease Drug
The Foundation in the NewsThe Foundation Fighting Blindness Retinal Degeneration Fund is an investor in the company
-

Oct 28, 2020
reVision Therapeutics Announces US FDA Grant of Rare Pediatric Disease and Orphan-Drug Designation for REV-0100 for the Treatment of Stargardt Disease
The Foundation in the News -
Sep 24, 2020
Foundation Fighting Blindness To Jointly Host Online Continuing Medical Education (CME, COPE) Webinar
Press ReleasesThe event will review best practices for care and management of patients with inherited retinal diseases such as retinitis pigmentosa, Usher syndrome, and Stargardt disease.
-
Aug 21, 2020
Foundation Fighting Blindness Commits $6.5 Million for New Retinal Disease Research Grants
Press ReleasesNew grants include development of CRISPR/Cas9 gene-editing treatments, new disease models, and a retinal regeneration therapy
-

Aug 4, 2020
Foundation Insights Forum – July 30, 2020
Insights ForumThe Foundation Fighting Blindness is pleased to provide an audio recording and full transcript of the Insights Forum, our quarterly conference call providing updates to the inherited retinal disease community. The call took place on July 30, 2020.
-
Mar 31, 2020
COVID-19 Resources
Foundation NewsThe Foundation Fighting Blindness is closely monitoring the COVID-19 situation and its impact on the IRD community.
-
Feb 6, 2020
Foundation Insights Forum – January 31, 2020
Insights ForumThe Foundation Fighting Blindness is pleased to provide an audio recording and full transcripts of the Insights Forum, our quarterly conference call providing updates to the inherited retinal disease community. The call took place on January 31, 2020.
-

Feb 6, 2020
ProQR Therapeutics Teams Up with the Foundation Fighting Blindness and Blueprint Genetics to Support the My Retina Tracker® Program for People Living with Inherited Retinal Diseases
Press ReleasesMy Retina Tracker Program is the highest volume IRD genetic testing program in the U.S.
-
Nov 8, 2019
Foundation Insights Forum – October 30, 2019
Insights ForumThe Foundation Fighting Blindness is pleased to provide an audio recording and full transcript of the Insights Forum, our quarterly conference call providing updates to the inherited retinal disease community. The call took place on October 30, 2019.
-
Oct 2, 2019
Blueprint Genetics, InformedDNA and the Foundation Fighting Blindness launch an open access program for patients with inherited retinal disease in the United States
Press ReleasesThe program will offer patients with inherited retinal disease no-cost genetic testing and genetic counseling in the United States. Look for updated information on how to participate to be posted in mid-October, with program registration starting shortly thereafter.
-
Aug 16, 2019
Foundation Fighting Blindness Investing Nearly $6.5 Million in New Grants
Foundation NewsThe newly funded research efforts include several therapies that have strong potential to treat a wide range of inherited retinal diseases.
-

May 9, 2019
Foundation Fighting Blindness Endorses 'Eye Bonds' Legislation
Press ReleasesBipartisan Bill Will Stimulate Up to $1 Billion in New Funding for Blindness Research
-

Jul 19, 2018
Foundation Fighting Blindness Urges Congress to Pass ‘Eye-Bonds’ Legislation
Press ReleasesBill Introduced in U.S. House Would Speed Up Cures for Blindness
-

Jun 15, 2018
Foundation Fighting Blindness Clinical Research Institute Hosted Workshop for ProgStar, the Largest Natural History Study for Stargardt Disease Ever Launched
Press ReleasesThe primary goal of the landmark study, funded by the Foundation, is to provide companies and researchers with disease-progression data and potential clinical trial endpoints to drive therapy development
-

Jun 8, 2018
Foundation Fighting Blindness and CheckedUp® Partner to Educate Retinal-Disease Patients About Research, Resources, and Emerging Therapies During Doctor Visits
Press ReleasesThe Foundation Fighting Blindness (the Foundation) and CheckedUp have formed a collaborative partnership to deliver patient-friendly diagnostic and disease-management information to people with retinal diseases such as age-related macular degeneration, retinitis pigmentosa, and Stargardt disease during their visits to eye doctors.
-
Apr 4, 2011
Researchers Take another Critical Step toward Using Skin Cells to Treat Retinal Disease
Foundation NewsA research team funded by the Foundation Fighting Blindness used an innovative repair technique to correct the disease-causing genetic defect in stem cells derived from a person’s skin — stem cells that hold the potential to treat their retinal degenerative condition.
Latest Research
-

Feb 1, 2024
Ascidian to Launch Clinical Trial for Stargardt Disease RNA Editing Therapy
Eye On the Cure Research NewsACDN-01 is the only genetic medicine entering clinical development for Stargardt disease.
-
Jan 8, 2024
Stargardt Disease Research Advances
Retinal Disease Research AdvancesRecent developments in research on Stargardt disease
-

Aug 25, 2022
Belite Bio Launching Phase 3 Clinical Trial for Stargardt Disease Drug
Eye On the Cure Research NewsThe company’s drug is designed to reduce accumulation of toxins that cause vision loss.
-

Jul 25, 2022
Nanoscope Doses First Patient in Phase 2 Clinical Trial of its Optogenetic Therapy for Stargardt Disease
Eye On the Cure Research NewsThe emerging therapy is designed to restore vision for people with advanced retinal degenerative diseases
-

May 2, 2022
Encouraging Clinical Trial Results for Alkeus’ Stargardt Disease Treatment
Eye On the Cure Research NewsThe company is also conducting a Phase 3 clinical trial of the emerging therapy for geographic atrophy.
-
Jan 7, 2022
Foundation’s RD Fund Invests in SalioGen Therapeutics, Developer of Novel Gene Coding Technology for Treating Inherited Retinal Diseases
Eye On the Cure Research NewsThe company is currently developing programs for Stargardt disease (ABCA4), Usher syndrome, RP25 (EYS), and RP1.
-
Apr 9, 2021
Foundation Invests $5.5 Million in Seven New Translational Research Projects
Eye On the Cure Research NewsProjects target a variety of conditions including: age-related macular degeneration, Stargardt disease, retinitis pigmentosa, and Usher syndrome type 3A
-

Feb 2, 2021
Very-Long-Chain Polyunsaturated Fatty Acids Improve Vision in Mice with a Form of Stargardt Disease
Eye On the Cure Research NewsResearchers believe the VLC-PUFAs may benefit people with autosomal dominant Stargardt disease, AMD, and possibly other retinal degenerations
-

Aug 3, 2020
Foundation Invests $3 million in Atsena Therapeutics, New Company Developing GUCY2D-LCA1 and MYO7A-USH1B Gene Therapies
Eye On the Cure Research NewsWith a founding investment from Hatteras Venture Partners, Atsena has raised a total of $8.15 million for its launch.
-

Jun 10, 2020
ProgStar Study Identifies Potential Endpoint for Clinical Trials of Emerging Stargardt Disease Treatments
Eye On the Cure Research NewsMicroperimetry, which measures retinal sensitivity in the macular region, shows promise as outcome measure
-

May 4, 2020
#GivingTuesdayNow Featured Researcher Dr. Shannon Boye
Eye On the Cure Research NewsA Lifelong Science Nerd is Winning the Fight Against Blindness
-

Feb 7, 2020
Genetic Testing for Inherited Retinal Diseases through the Foundation’s Open Access Program
Science EducationThe benefits of genetic testing for IRD patients, how to participate in the Foundation’s Open Access program, and what to expect from the genetic testing process.
-

Nov 7, 2019
AGTC Announces Development of Stargardt Disease Gene Therapy
Eye On the Cure Research NewsDual-vector delivery system designed to deliver the large ABCA4 gene
-

Oct 29, 2019
Iveric Bio’s Therapy Slows Retinal Degeneration in Phase 2b Trial for Dry AMD
Eye On the Cure Research NewsZimura inhibits a complement protein known as C5, a component of the immune system that, when overactive, can cause retinal degeneration.
-
Jun 17, 2019
The Retina is a Proving Ground for a Broad Range of Neurological Therapies
Science EducationRetinal research paves the way for new treatments for the entire neurological system.
-

May 20, 2019
Dr. Don Zack Honored for Research Contributions by ARVO and the Foundation Fighting Blindness
Eye On the Cure Research NewsDr. Zack is a member of the Foundation’s Scientific Advisory Board and chairs its Cellular Molecular Mechanisms of Disease study section.
-

May 9, 2019
Eye Bonds Re-Introduced to New Congress: Potentially $1 Billion in Government-Backed Funding for Eye Research
Eye On the Cure Research NewsEye Bonds provide the opportunity to advance, and accelerate development for, more promising treatments into and through clinical trials and out to the people who need them.
-

Jan 29, 2019
The Foundation Receives a $100,000 Research Grant from Sofia Sees Hope
Eye On the Cure Research NewsSofia Sees Hope, a nonprofit dedicated to finding treatments and cures for people with Leber congenital amaurosis (LCA) and other inherited retinal diseases (IRDs), has made a $100,000 donation to the Foundation Fighting Blindness to support therapy development and genetic testing.
-
Jan 17, 2019
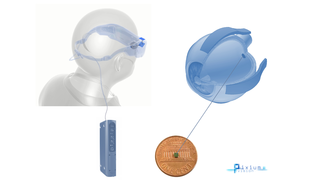
Pixium's PRIMA Bionic Vision System Restores Central Vision in Dry AMD Clinical Trial
Eye On the Cure Research NewsThe French bioelectronics company Pixium Vision has reported that its PRIMA bionic vision system has restored some central vision in patients with advanced dry age-related macular degeneration (AMD) participating in a clinical feasibility trial.
-

Nov 21, 2018
Acucela Enrolling Patients in Phase 3 Trial for Stargardt Disease Treatment
Eye On the Cure Research NewsThe Seattle biotech Acucela is now enrolling participants in its Phase 3 clinical trial for emixustat hydrochloride, an emerging oral drug for slowing vision loss in people with Stargardt disease, an inherited form of a macular degeneration.
-

Nov 2, 2018
Foundation Invests $2.5 Million in Search for Elusive Retinal Disease Genes and Mutations
Eye On the Cure Research NewsSince 1989 genetic researchers, many funded by the Foundation, have identified approximately 270 genes linked to IRDs. In most cases, defects in a single gene can cause a retinal disease and vision loss.
-

Sep 11, 2018
FFB Congratulates RPE65 Gene Therapy Researchers for Champalimaud Award
Eye On the Cure Research NewsOn September 4, 2018, seven researchers, including six previously funded by the Foundation, were recognized with the prestigious 2018 Antonio Champalimaud Vision Award for their contributions to the advancement of blindness-reversing RPE65 gene therapies.
-

Aug 22, 2018
Ophthotech is Advancing an Impressive Portfolio of Cutting-Edge Therapies for Retinal Diseases
Eye On the Cure Research NewsThe company is taking on a multi-track strategy that includes retinal gene-therapy development, including delivery of over-sized genes and design of a two-step process of gene knockdown and replacement for autosomal dominant conditions.
-
Aug 15, 2018
FFB Provides Four Career Development Awards to Up-and-Coming Clinical Researchers
Eye On the Cure Research NewsEach recipient will receive a total of $375,000 over five years to help build an independent research program in addition to their clinical practices.
-
Aug 6, 2018
FFB Funding More than $2 Million in New Research
Eye On the Cure Research NewsSeventy scientists submitted requests for funding.
-

Jul 20, 2018
Call to Action: Ask Congress to Support $1 Billion in Eye Research
Eye On the Cure Research NewsCall to Action: Ask Congress to Support $1 Billion in Eye Research
-
Jul 5, 2018
Retinal Regeneration: Releasing Your Inner Salamander
Eye On the Cure Research NewsMany research groups from around the world are investigating ways to create new photoreceptors from stem cells for transplantation into the retina for vision restoration.
-
Jun 22, 2018
VISIONS2018 Live Stream
Eye On the Cure Research NewsWatch recorded sessions from VISIONS2018.
-

May 3, 2018
ARVO 2018: Dr. Stephen Daiger Reports on the State of Genetic Testing for Inherited Retinal Diseases
Eye On the Cure Research NewsARVO 2018: Dr. Stephen Daiger Reports on the State of Genetic Testing for Inherited Retinal Diseases
-

May 2, 2018
ARVO 2018: Dr. Steve Rose Reports on CRISPR/Cas9 for Inherited Retinal Diseases
Eye On the Cure Research NewsFFB’s own Dr. Steve Rose, chief scientific officer, reviews our commitment to funding and exploring CRISPR/Cas9 gene editing for inherited retinal disease.
-

Apr 25, 2018
ARVO 2018: World's Largest Show and Tell for Innovations in Eye Research
Eye On the Cure Research NewsMore than 11,000 eye researchers from around the world — including five intrepid members from FFB’s science team — will gather to participate in what is essentially a massive “show and tell” of the latest scientific advancements.
-

Jan 23, 2018
Ophthotech Launching Human Study of Emerging Therapy for Stargardt Disease
Eye On the Cure Research NewsOphthotech, a biopharmaceutical company developing therapies for eye diseases, has enrolled the first patient in its Phase 2b clinical trial of Zimura® for people with Stargardt disease caused by mutations in the gene ABCA4.
-
Jan 17, 2018
Clinical Trial to Launch for System Combining Optogenetics and Eyewear
Eye On the Cure Research NewsThe French biotech GenSight Biologics has received regulatory authorization in the UK to launch the PIONEER Phase 1 \ 2 clinical trial for its GS030 system — a light-sensing gene therapy (optogenetics) coupled with eyewear, which enhances visual stimulation.
-

Jan 9, 2018
Top Retinal Research Advances for 2017
Eye On the Cure Research NewsAn exciting year in fighting blindness.
-
Dec 20, 2017
History Is Made: FDA Approves Spark's Vision-Restoring Gene Therapy
Eye On the Cure Research NewsKnown as LUXTURNA™ (voretigene neparvovec), the gene therapy restored vision in a clinical trial for people between the ages of 4 and 44 with Leber congenital amaurosis (LCA) caused by mutations in the gene RPE65.
-
Nov 21, 2017
Stem-Cell Therapy Clinics Remain Inadequately Regulated, Pose Risk to Patients
Eye On the Cure Research NewsIf a clinic is charging for a stem-cell treatment or procedure for an IRD, it is probably not legit. The expense to the patient is a major red flag.
-

Oct 13, 2017
FDA Committee Unanimously Recommends Approval for Spark's RPE65 Gene Therapy - Final Decision Due in January 2018
Eye On the Cure Research NewsAn advisory committee comprised of FDA-selected experts voted unanimously – 16 to 0 – to recommend approval.
-

Sep 27, 2017
The Foundation's Investments Are Filling the Pipeline for Vision-Saving Therapies
Eye On the Cure Research NewsIn addition to funding promising biotech start-ups, the Foundation Fighting Blindness has played a critical role in developing research talent.
-
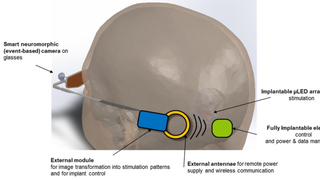
Jul 26, 2017
Scientists Receive $25 Million to Develop a Vision-Restoring System that Connects to the Brain
Eye On the Cure Research NewsThe high-tech, vision-restoring system interfaces with the visual cortex, the back of the brain where visual input is processed to create the images we see.
-
Jul 25, 2017
Foundation Fighting Blindness and 4D Molecular Therapeutics Partner to Boost Retinal Gene Therapy Development
Eye On the Cure Research NewsThe partnership will help companies and researchers quickly obtain and implement high-quality vectors for their retinal gene-therapy development efforts.
-
Jul 24, 2017
FFB-Funded Scientists Report on Nine Promising Translational Research Efforts
Eye On the Cure Research NewsThe Foundation Fighting Blindness has taken the translational challenge head on by investing more than $75 million in therapy-development projects with strong clinical-trial potential through its Translational Research Acceleration Program (TRAP), which includes Gund-Harrington Scholar Awards.
-

May 23, 2017
Forty-Four High-Impact Retinal-Research Efforts Highlighted at FFB-Casey Innovation Summit
Eye On the Cure Research NewsIn its fourth year, the meeting is becoming the world’s most comprehensive overview of the promising research underway for emerging IRD treatments.
-

May 8, 2017
FFB Funding Helps Retinal Genetics Lab Secure $2 Million Investment
Eye On the Cure Research NewsHow the Foundation Fighting Blindness (FFB) provided timely funding of $155,000 to help a lab at the University of California, San Diego (UCSD), leverage a $2 million retinal-gene discovery project.
-

Mar 22, 2017
Dr. Eliot Berson, Pioneer in Vitamin A Therapy for Retinitis Pigmentosa, Passes Away
Eye On the Cure Research NewsDr. Berson dedicated himself to clinical care and vision-saving research for people with inherited retinal diseases for five decades.
-
Mar 16, 2017
Unregulated Stem-Cell Therapy Causes Severe Vision Loss for Three Florida Women
Eye On the Cure Research News“…participation in a study for an emerging therapy that is not regulated by the FDA or another well-recognized regulatory agency like the European Medicines Agency in Europe, is fraught with dangers and can lead to unexpected serious consequences.”
-
Feb 28, 2017
FFB Convenes Experts to Discuss Therapeutic Opportunities for Stargardt Disease
Eye On the Cure Research NewsMuch of the information and data shared during the meeting came out of ProgSTAR, the FFB-CRI-funded natural history study of 365 Stargardt disease patients.
-
Feb 17, 2017
AGTC Leverages Funding from the Foundation to Move Promising Treatments into Clinical Trials
Eye On the Cure Research NewsCompany Builds on FFB’s Initial Investment to Garner $265 Million in Therapy Development Funding
-
Dec 21, 2016
FFB-CRI Leads Effort to Identify Outcome Measures for Therapies in Clinical Trials
Eye On the Cure Research NewsImproved outcome measures will make clinical trials for degenerative retinal diseases — including age-related macular degeneration (AMD), the world’s leading cause of blindness in seniors, and inherited retinal conditions such as RP and Stargardt disease — less expensive to conduct and able to deliver more precise results.
-
Oct 18, 2016
Building a Wiring Diagram for the Retina to Help Researchers Save and Restore Vision
Eye On the Cure Research NewsUnderstanding the pathways of the retinal neural network — and how they are rewired with aging and disease — is helpful in trying to save and restore vision.
-

Oct 11, 2016
Nobel-Prize-Winning Stem-Cell Researcher Delivers Keynote at FFB-Funded Conference in Kyoto
Eye On the Cure Research NewsDr. Shinya Yamanka discussed his early clinical trial for iPSC-derived retinal pigment epithelial (RPE) cells for a 78-year-old woman with advanced wet age-related macular degeneration (AMD).
-
.png)
Oct 6, 2016
Embrace Your Exceptions: A Mantra for Understanding Retinal-Disease Inheritance
Eye On the Cure Research NewsThe complex and elusive nature of these conditions can also extend to the way they are passed down in families, making diagnosis and prognosis quite challenging.
-

Aug 18, 2016
Optogenetic Therapy Takes First Step Forward in Clinical Trial
Eye On the Cure Research NewsRetroSense’s optogenetic therapy is designed to restore vision to people who are completely blind from retinal degenerative diseases such as retinitis pigmentosa by bestowing light sensitivity to retinal ganglion cells, which survive after photoreceptors, the cells that make vision possible, are lost.
-

Aug 2, 2016
Pixium Vision Reports Progress in Development of Two Advanced Bionic Retina Systems
Eye On the Cure Research NewsBoth approaches show strong, near-term potential for providing meaningful vision to people who are otherwise blind from retinal diseases such as retinitis pigmentosa and age-related macular degeneration (AMD).
-

Jul 1, 2016
VISIONS 2016 - Dr. Richard Weleber Receives FFB's Highest Research Honor, Recognized in Touching Video
Eye On the Cure Research NewsDr. Weleber became the 10th recipient of the Foundation’s highest honor, named after FFB co-founder Lulie Gund, during the opening lunch of the VISIONS 2016 conference.
-
Jul 1, 2016
VISIONS 2016 — Dr. Shomi Bhattacharya Wins FFB Award for Gaining an Understanding of Variations in Vision Loss
Eye On the Cure Research NewsAt VISIONS 2016, FFB’s national conference, the Foundation honored him with its Ed Gollob Board of Directors Award for breakthrough research conducted within the past year.
-

Jun 27, 2016
Two Philanthropic Brothers — Selling Luxury Casual Wear for the Good of Retinal Research
Science EducationThe story of Two Blind Brothers, a luxury clothing line that donates its proceeds to retinal research.
-
Jun 24, 2016
A Steady Hand in Saving Vision
Eye On the Cure Research NewsSubretinal injection is the most common form of delivery for gene therapies currently in clinical trials.
-

May 4, 2016
ARVO 2016: Emerging Drug Targets Toxic Build-Up in Stargardt Disease
Eye On the Cure Research NewsIn August 2015, the biotech company Alkeus launched a multi-center TEASE Phase II clinical trial for the drug ALK-001.
-

May 2, 2016
ARVO 2016: ProgSTAR, FFB-CRI's Stargardt Disease Patient Study, Highlighted
Eye On the Cure Research NewsWhen the study and the reports are completed, the ProgSTAR database will be made available to researchers and companies developing therapies.
-

Oct 8, 2015
A Leap Forward: Spark Therapeutics Seeks FDA Approval for its Vision-Restoring Gene Therapy
Eye On the Cure Research News -
Jun 27, 2015
VISIONS 2015 — Dr. José Sahel Receives Foundation's Most Prestigious Research Honor
Eye On the Cure Research NewsFor those of us supporting the drive for vision-saving treatments and cures, he’s exactly the type of person we want on our team.
-

Jun 26, 2015
VISIONS 2015 — Dr. Shannon Boye Receives FFB Award for Excellence in Gene-Therapy Research
Eye On the Cure Research NewsDr. Boye received the Foundation’s Board of Director’s Award, which was presented at VISIONS 2015, FFB’s annual conference, for achievements in retinal research.
-
May 19, 2015
ARVO 2015 Highlight: The National Eye Institute Invests $4 Million in Audacious-Goals Research
Eye On the Cure Research NewsThe mission of the program—to regenerate the neurons and neural connections in the eye and visual system—is synonymous with the Foundation’s mission to eradicate retinal diseases.
-
May 12, 2015
ARVO 2015 Highlight: A Cut-and-Paste Approach to Fixing Retinal-Disease Genes
Eye On the Cure Research NewsOne of the hot topics at ARVO this year is a rapidly advancing gene-therapy approach called clustered regularly interspaced short palindromic repeats, or CRISPR.
-

Aug 1, 2014
How Evolution is Leading to Gene Therapies for More Retinal Diseases
Eye On the Cure Research NewsAn innovative genetic-engineering approach called “directed evolution” to find optimal gene-delivery systems based on adeno-associated viruses (AAVs).
-
Jun 21, 2014
VISIONS 2014 — The Multi-Talented Dr. Shannon Boye
Eye On the Cure Research NewsDr. Boye and her research team received a $900,000 grant for a gene therapy project targeting Leber congenital amaurosis.
-
Jun 21, 2014
VISIONS 2014 — My Retina Tracker: Track Your Vision and Drive the Research
Eye On the Cure Research NewsThe powerful and secure system enables patients to keep track of their clinical care and vision changes. At the same time, it enables scientists to search the “de-identified” (i.e., anonymous) patient information to study conditions and identify targets for treatments, preventions and cures.
-
May 8, 2014
ARVO 2014: European Collaboration Developing Cross-Cutting, Vision-Saving Therapies
Eye On the Cure Research NewsSimply put, they’re creating therapies that can save vision in as many people as possible, independent of the genetic cause of disease.
-
Apr 8, 2014
Total Blindness and Non-24 Sleep Disorder
Eye On the Cure Research NewsNon-24 is a very rare condition affecting many (but not all) people who are totally blind and have absolutely no light perception. Their circadian clocks become out of sync as a result.
-
Dec 31, 2013
Nouvelle Lumière: French Bionic Retina in a Human Study
Eye On the Cure Research NewsThe French retinal implant developer Pixium quietly launched a clinical trial for its Intelligent Retinal Implant System 1 (IRIS1) in France, Austria and Germany.
-
Jul 26, 2013
Researchers Move Closer to Getting a Complete Genetic Picture of the Retina
Eye On the Cure Research NewsIdentifying the genes and proteins that play a major role in retinal health and vision is an important step in finding preventions and cures for degenerative diseases.
-
Jun 12, 2013
Patient Registries Help Advance Research for Rare Diseases
Eye On the Cure Research NewsMany registries enable patients to collect and track information about their health, so they can take an active role in managing their care.
-
May 10, 2013
Grow Your Own: Harnessing Muller Glia for Retinal Regeneration
Eye On the Cure Research NewsThere’s hope for retinal regeneration for humans, thanks to Foundation-funded researcher Dr. Thomas Reh, who is investigating how to derive new photoreceptors from retinal cells called Muller glia.
-
May 7, 2013
Retinal Regeneration is Major Focus of NEI's Audacious Goal
Eye On the Cure Research NewsThe goal, “to regenerate the neurons and neural connections in the eye and visual system,” is exactly what people with retinal diseases need to save and restore their vision.
-

Apr 30, 2013
Researcher Revolutionized Fight Against Blindness and Cancer
Eye On the Cure Research NewsA profile on Dr. Robert Langer, a medical researcher who has received dozens of awards, accolades and honorary degrees, including, recently, FFB’s Visionary Award.
-

Mar 29, 2013
DHA and EPA for Stargardt Disease — an Evolving Story
Eye On the Cure Research NewsThese healthy fats have exciting potential for treating a wide range of retinal degenerations and other health conditions.
-

Mar 15, 2013
Proving a Vision-Saving Treatment Works
Science EducationThe information gleaned from ProgSTAR will be of enormous value in designing future clinical trials for Stargardt disease treatments.
-
Mar 8, 2013
Staying Alive: Saving Retinal Cells to Preserve Vision
Science EducationSometimes, saving vision simply comes down to keeping retinal cells alive, or at least slowing their degeneration.
-

Feb 18, 2013
History in the Making
Eye On the Cure Research NewsMore good news about treatments and technological advances for restoring vision for people with retinal diseases.
-
Jun 19, 2012
Have I Got a Cure for You! Debunking an Alleged Treatment on the Internet
Eye On the Cure Research NewsHow do you know if a treatment is legit? There should be preclinical and clinical trial data published in a peer-reviewed journal on research for the treatment.
-

Jan 26, 2011
Acucela Initiates Phase 2a Study of Emixustat Hydrochloride Addressing Patients with Stargardt Disease
Eye On the Cure Research NewsThe study is designed to evaluate the pharmacodynamics, safety and tolerability of emixustat in subjects with macular atrophy secondary to Stargardt disease.
Related Resources
-

Mar 25, 2024
Rocking On With Stargardt
Beacon Stories DIY Campaign Success StoriesAdopted into a musical family, Miles Hoyt picked up a guitar at just four years old, and he hasn’t stopped playing since. Now Miles, who has Stargardt disease, and his parents are using music to bring their community together to raise funds for blinding diseases with their Raising Our Sights fundraiser, Smiles for Miles.
-

Mar 11, 2024
Influencing Positivity for the Disabled Community
Beacon StoriesContent creator and social media influencer Stephanie Renberg uses her platforms to raise awareness for blindness and disabilities in hopes that her openness about her Stargardt disease will contribute to a more inclusive and understanding world.
-

Jul 24, 2023
Chloe’s Creative Journey with Vision Loss
Beacon StoriesChloé Duplessis is a legally blind digital collage artist in Denver, Colorado. At 39 years old, Chloé was diagnosed with Stargardt disease, which was a pivotal point in her creative journey to becoming a full-time artist and owner of Duplessis Art.
-

Mar 27, 2023
Evolving With Eyesight and Life
Beacon StoriesGavin was diagnosed with Stargardt disease at the age of 19. In his own words, Gavin shares his experience over the last 13 years adjusting to the unpredictability of his vision loss and how being a new parent has shifted his mindset.
-

Jul 25, 2022
Discovering Confidence Through Vulnerability
Beacon StoriesFashion and Lifestyle Blogger Lindi Goff is sharing her authentic self by being vulnerable about her journey with Stargardt disease.
-
.jpg)
Jul 11, 2022
Eye on the Prize Winner: Josh/Nicholls State University
Beacon Stories DIY Campaign Success StoriesThis year, the Foundation created a new fundraising initiative called Eye on the Prize, influenced by March Madness. The winner of the inaugural Eye on the Prize competition was Nicholls State University, raising $2,428. This winning team was led by Josh Cogswell, an assistant professor of management at Nicholls State University, who has his own personal connection to the Foundation’s mission.
-

Apr 26, 2022
“Great Vision Does Not Require Great Sight”
Beacon StoriesKenyetta has worked to help others through the nonprofit sector for over 25 years, despite being diagnosed with Stargardt disease at the age of 31. Kenyetta is now the chief operating officer of REACH Riverside Development Corporation and has been recognized for her career achievements by many.
-

Jan 10, 2022
Ambitious Entrepreneur Won’t Let Stargardt Stop Her
Beacon StoriesBeverley is a go-getter who does not let her Stargardt disease get in the way of living her life to the fullest. When Beverley isn’t hard at work as an entrepreneur, she enjoys spending time with friends, her dog, Loki, and is also an active leader of the Foundation’s Boston Chapter.
-
.jpg)
Jun 21, 2021
Blind YouTuber Finding His Spot in Life
Beacon StoriesYouTuber Sam Seavey was diagnosed with Stargardt disease when he was just 11 years old. Sam didn’t fully accept his visual impairments until his early 30s. Now he’s helping others through “The Blind Life,” which is currently the most extensive resource for assistive technology on the internet.
-

Jun 15, 2020
Jumping into an Uncharted Arena
Beacon StoriesThirty-one-year-old Wren Blae Zimmerman always loved horses. But it wasn’t until a few years ago that Wren finally learned to ride. Now her life revolves around this equestrian dream, and she’s given herself the title of the “Blind Show Jumper.”
-

Mar 23, 2020
No Limits for Determined Paralympian Triathlete
Beacon StoriesParalympian Elizabeth Baker says that Stargardt disease has made her a tougher person.
-

Nov 22, 2019
A Renegade on a Mission
Beacon StoriesDr. Marciello doesn’t let a Stargardt disease diagnosis get in the way of his twin passions: medicine and baseball.
-

Oct 28, 2019
Visionary Artist Elijah Overcomes All Obstacles
Beacon StoriesElijah has always had an artist mind, and he hasn’t let his diagnosis with Stargardt disease hold him back from his passion for creating art.
-
.png)
Sep 30, 2019
The Show Must Go on Despite Vision Loss Due to Stargardts
Beacon StoriesEmmy Award-Winning Writer and Performer Ellen Gould Weaves her Personal Experience with Stargardts Into Her New Musical, “Seeing Stars”
-

Jul 27, 2018
Persevering to Success with the Support of Family, Friends, and Faith
Beacon StoriesA story about living with retinitis pigmentosa.
-

Sep 20, 2017
Heather Presnar’s Story: Living with Stargardt Disease
Beacon StoriesWhen you’re the catcher, the ball always comes to you. Heather Presnar wouldn’t have had it any other way.
-

Jun 27, 2016
Two Philanthropic Brothers — Selling Luxury Casual Wear for the Good of Retinal Research
Beacon Stories -

May 30, 2012
Worldwide Vision
Beacon StoriesBrooke James has had more than a glimpse of what it means to be blind in certain parts of the world. And she can assure you, it’s scary.




