Leber Congenital Amaurosis (LCA)
LCA is a group of inherited retinal diseases causing blindness or severe vision loss in early childhood.
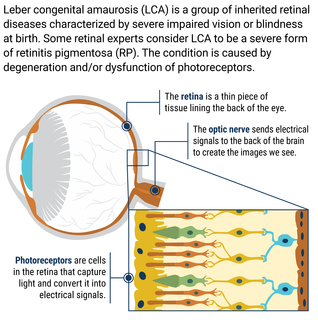
What is Leber Congenital Amaurosis?
Leber congenital amaurosis (LCA) is a group of inherited retinal diseases characterized by severe impairment vision or blindness at birth. Some retinal experts consider LCA to be a severe form of retinitis pigmentosa (RP). The condition is caused by degeneration and/or dysfunction of photoreceptors, the cells in the retina that make vision possible. Photoreceptors capture light, converting it to electrical signals which are sent to the back of the brain to create the images we see. Mutations in one of more than two dozen genes can cause LCA.
Symptoms
Often within an affected infant’s first few months of life, parents notice a lack of visual responsiveness and roving eye movements, known as nystagmus. Eye examinations of infants with LCA sometimes reveal normal-appearing retinas. In other cases, several abnormalities are observed. Regardless, an electroretinogram (ERG), which measures retinal function, detects little if any activity in the retina. ERG tests are often essential to establishing a diagnosis of LCA. A genetic test can often provide a definitive diagnosis.
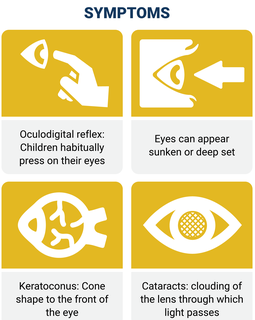
Many children with LCA habitually press their eyes with their fists or fingers. This habitual pressing on the eyes is known clinically as oculodigital reflex. The eyes of individuals with LCA can also appear sunken or deep set. Keratoconus (cone shape to the front of the eye) and cataracts (clouding of the lens through which light passes) can occur with the disease.
In some cases, other body systems (e.g., kidneys) can be affected by the genetic defects that cause LCA.
The most common genes associated with Leber congenital amaurosis (LCA) are CEP290, CRB1, GUCY2D, and RPE65.
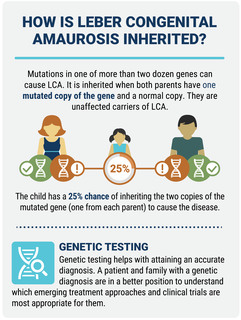
Inheritance
LCA is almost always passed down through the autosomal recessive pattern of inheritance. In this type of inheritance, both parents, called carriers, have one mutated copy of the gene and one normal gene. They are unaffected carriers of LCA. Each of their children has a 25 percent chance of inheriting the two LCA gene copies (one from each parent) needed to cause the disorder.
Living with LCA
There are many services and accommodative and assistive resources available to people and families with LCA. Visit the Foundation’s Low Vision Resources page to learn about many of these resources. A low vision specialist can help recommend the resources that are right for you.
More information on managing LCA is in the Newly Diagnosed section of this Web site.
Genetic Testing
Genetic testing is available for LCA, and helps with attaining an accurate diagnosis. A patient and family with a genetic diagnosis are in a better position to understand which emerging treatment approaches and clinical trials are most appropriate for them.
Treatments
In December 2017, the biotech Spark Therapeutics obtained U.S. Food and Drug Administration approval for LUXTURNA™, an RPE65 gene therapy that has improved vision in children and young adults with RPE65 mutations. Early funding from the Foundation helped make this treatment possible. Treatments for forms of LCA caused by other mutated genes are in the development pipeline.
Research and Clinical Trials
For the latest research advances for LCA, refer to the Foundation publication: Leber Congenital Amaurosis: Research Advances.
View a list of current clinical trials, many made possible by Foundation support.
LCA page at www.ClinicalTrials.gov.
Next Section
Read the Most Recent Research on Leber Congenital Amaurosis (LCA)
Latest News
-

Mar 25, 2024
BlueRock Therapeutics and Foundation Fighting Blindness announce collaboration to expand the Uni-Rare natural history study of patients living with inherited retinal diseases
Press ReleasesCollaboration will add a new multi-gene cohort of patients living with inherited retinal diseases. Data insights from the new study cohort will inform the future clinical trial design for BlueRock’s pipeline of cell therapies for treating blindness.
-

Nov 10, 2022
Foundation Launching its Largest Natural History Study to Date for 1,500 People with Inherited Retinal Diseases Caused by Rare Mutated Genes
Press ReleasesUni-Rare Study will improve clinical understanding of more IRDs and boost development of potential therapies.
-

May 12, 2022
Opus Genetics Appoints Jennifer Hunt Chief Development Officer
The Foundation in the NewsBiopharma clinical development veteran to propel Opus’ AAV-based gene therapies for inherited retinal diseases toward patients.
-

Sep 22, 2021
RD Fund Launches Opus Genetics with $19M Seed Funding to Advance Gene Therapy Treatments for Blinding Conditions
The Foundation in the NewsInitial programs will focus on treatments for rare pediatric blinding conditions. Company formed to advance the work of scientific cofounders Dr. Jean Bennett, Junwei Sun and Dr. Eric Pierce.
-

Dec 16, 2020
Atsena Therapeutics Raises $55 Million Series A Financing to Advance LCA1 Gene Therapy Clinical Program, Two Preclinical Assets, and Novel Capsid Development for Ocular Diseases
The Foundation in the NewsRound was led by Sofinnova Investments with participation from Abingworth, Lightstone Ventures and all existing investors.
Company expands board of directors and plans to build out team.
-

Aug 12, 2020
X Ambassadors Teams up with Foundation Fighting Blindness and Two Blind Brothers to Launch "Music to Our Eyes" Exclusive Livestream Music Series
Press ReleasesThis exclusive conversation and acoustic performance by Sam and Casey Harris of X Ambassadors on August 20, will raise awareness and funds to find treatments and cures for blinding diseases.
-

Aug 4, 2020
Foundation Insights Forum – July 30, 2020
Insights ForumThe Foundation Fighting Blindness is pleased to provide an audio recording and full transcript of the Insights Forum, our quarterly conference call providing updates to the inherited retinal disease community. The call took place on July 30, 2020.
-

Jul 29, 2020
Atsena Therapeutics acquires exclusive rights to Gene Therapy for GUCY2D-associated Leber Congenital Amaurosis
The Foundation in the NewsCompany formed with $8.15 million Series 1; led by founding investors Hatteras Venture Partners and the Foundation Fighting Blindness
-
Mar 31, 2020
COVID-19 Resources
Foundation NewsThe Foundation Fighting Blindness is closely monitoring the COVID-19 situation and its impact on the IRD community.
-
Mar 12, 2020
Seeing hope: Ledyard nonprofit focuses on rare retinal diseases
NewsSofia Priebe, 17, is legally blind. Her parents have started a nonprofit, Sofia Sees Hope, to raise funds for research into and awareness of the rare genetic disease Leber congenital amaurosis, which causes her blindness.
-
Feb 6, 2020
Foundation Insights Forum – January 31, 2020
Insights ForumThe Foundation Fighting Blindness is pleased to provide an audio recording and full transcripts of the Insights Forum, our quarterly conference call providing updates to the inherited retinal disease community. The call took place on January 31, 2020.
-

Feb 6, 2020
ProQR Therapeutics Teams Up with the Foundation Fighting Blindness and Blueprint Genetics to Support the My Retina Tracker® Program for People Living with Inherited Retinal Diseases
Press ReleasesMy Retina Tracker Program is the highest volume IRD genetic testing program in the U.S.
-
Nov 8, 2019
Foundation Insights Forum – October 30, 2019
Insights ForumThe Foundation Fighting Blindness is pleased to provide an audio recording and full transcript of the Insights Forum, our quarterly conference call providing updates to the inherited retinal disease community. The call took place on October 30, 2019.
-
Oct 2, 2019
Blueprint Genetics, InformedDNA and the Foundation Fighting Blindness launch an open access program for patients with inherited retinal disease in the United States
Press ReleasesThe program will offer patients with inherited retinal disease no-cost genetic testing and genetic counseling in the United States. Look for updated information on how to participate to be posted in mid-October, with program registration starting shortly thereafter.
-
Aug 16, 2019
Foundation Fighting Blindness Investing Nearly $6.5 Million in New Grants
Foundation NewsThe newly funded research efforts include several therapies that have strong potential to treat a wide range of inherited retinal diseases.
-

Jul 19, 2018
Foundation Fighting Blindness Urges Congress to Pass ‘Eye-Bonds’ Legislation
Press ReleasesBill Introduced in U.S. House Would Speed Up Cures for Blindness
-

Jun 8, 2018
Foundation Fighting Blindness and CheckedUp® Partner to Educate Retinal-Disease Patients About Research, Resources, and Emerging Therapies During Doctor Visits
Press ReleasesThe Foundation Fighting Blindness (the Foundation) and CheckedUp have formed a collaborative partnership to deliver patient-friendly diagnostic and disease-management information to people with retinal diseases such as age-related macular degeneration, retinitis pigmentosa, and Stargardt disease during their visits to eye doctors.
-

Jan 9, 2018
A Retinal Research Nonprofit Paves the Way for Commercializing Gene Therapies
The Foundation in the NewsAN EMERGING, vision-restoring gene therapy for a devastating retinal disease is poised for Food and Drug Administration (FDA) approval. If it gets the regulatory nod, it will be the first gene therapy to receive FDA approval for the eye or an inherited condition.
Latest Research
-

Mar 26, 2024
Opus Reports Vision Improvements for Patients in LCA5 Gene Therapy Clinical Trial
Eye On the Cure Research NewsThe company plans to administer a higher dose of the emerging gene therapy to the next group of patients.
-

Jan 8, 2024
Leber Congenital Amaurosis Research Advances
Retinal Disease Research AdvancesRecent developments in research on Leber congenital amaurosis
-

Dec 11, 2023
Théa Completes Acquisition of ProQR’s LCA10 and USH2A Treatment Programs, Plans to Continue Clinical Development
Eye On the Cure Research NewsKnown as antisense oligonucleotides, the treatments performed encouragingly in ProQR’s clinical trials
-

Sep 7, 2023
First Patient Dosed in LCA5 Gene Therapy Clinical Trial Launched by Opus Genetics
Eye On the Cure Research NewsOpus was established by the RD Fund, the Foundation’s venture philanthropy arm
-

Jun 29, 2023
University of Wisconsin-Madison Awarded $29 Million NIH Common Fund Grant to Develop LCA16 and Best Disease Gene-Editing Therapies
Eye On the Cure Research NewsThe five-year grant will advance the emerging treatments toward clinical trials
-

May 1, 2023
Eric Pierce Receives Proctor Medal for Outstanding Achievements in Retinal Research
Eye On the Cure Research NewsDuring his Proctor Award Lecture, Dr. Pierce reviewed encouraging clinical trial results for Editas’ CRISPR/Cas9 treatment for people with LCA10
-

Dec 2, 2022
Opus Genetics to Launch Gene Therapy Clinical Trial for LCA5 Patients
Eye On the Cure Research NewsThe LCA5 gene therapy will be the first emerging Opus treatment to move into a human study
-

Nov 18, 2022
Editas Reports Results for 14 Participants in its Phase 1/2 CRISPR/Cas9 Clinical Trial for LCA10
Eye On the Cure Research NewsThe company is seeking a partner to move the LCA10 program forward
-

Oct 3, 2022
Atsena’s LCA-GUCY2D Gene Therapy Improves Vision in Phase 1/2 Clinical Trial
Eye On the Cure Research NewsThe company is planning to move the emerging treatment into a pivotal trial
-

Aug 11, 2022
ProQR Seeking to Partner Ophthalmic Programs
Eye On the Cure Research NewsThe company is halting its clinical programs for LCA10 and USH2A as it seeks a new partner
-

May 19, 2022
ProQR’s Sepofarsen Improves Vision Significantly for Girl with LCA10
Eye On the Cure Research NewsHowever, the Phase 2/3 Illuminate trial for sepofarsen didn’t meet its endpoints at 12 months
-

Feb 15, 2022
ProQR’s Phase 2/3 Clinical Trial for LCA10 RNA Therapy Doesn’t Meet Endpoints
Eye On the Cure Research NewsThe emerging treatment had shown encouraging results in a Phase ½ clinical trial
-

Sep 30, 2021
Vision Improvements Reported for Two LCA 10 Patients in Phase 1/2 Clinical Trial for Editas’ CRISPR/Cas9 Treatment
Eye On the Cure Research NewsThe emerging treatment targets a specific mutation (c.2991+1655A>G in Intron 26) of the gene CEP290 which causes Leber congenital amaurosis 10 (LCA 10)
-

Apr 12, 2021
Encouraging Early Report for Three Patients in LCA1-GUCY2D Gene Therapy Clinical Trial
Eye On the Cure Research NewsDevelopment of the emerging gene therapy is being funded by the Foundation’s RD Fund
-

Jan 13, 2021
ProQR Completes Enrollment in Phase 2/3 Clinical Trial of RNA Therapy for LCA10
Eye On the Cure Research NewsCompany also announced progress in enrollment for Phase 1 / 2 clinical trials for emerging USH2A and RHO RNA therapies.
-

Aug 3, 2020
Foundation Invests $3 million in Atsena Therapeutics, New Company Developing GUCY2D-LCA1 and MYO7A-USH1B Gene Therapies
Eye On the Cure Research NewsWith a founding investment from Hatteras Venture Partners, Atsena has raised a total of $8.15 million for its launch.
-

May 4, 2020
#GivingTuesdayNow Featured Researcher Dr. Shannon Boye
Eye On the Cure Research NewsA Lifelong Science Nerd is Winning the Fight Against Blindness
-
Mar 5, 2020
First Patient Receives Emerging CRISPR Therapy in Clinical Trial for LCA 10
Eye On the Cure Research NewsFirst time emerging CRISPR therapy administered inside the human body
-

Mar 5, 2020
ARVO 2014: LCA Gene Therapy Recipient Featured During Keynote
Eye On the Cure Research News15-year-old Yannick Duwe transfixed the audience, describing how the treatment enabled him to use a computer instead of Braille, and work much easier and faster at school.
-

Feb 7, 2020
Genetic Testing for Inherited Retinal Diseases through the Foundation’s Open Access Program
Science EducationThe benefits of genetic testing for IRD patients, how to participate in the Foundation’s Open Access program, and what to expect from the genetic testing process.
-

Dec 11, 2019
Families and Scientists Meet to Discuss Therapy Development for People with RDH12 Mutations
Eye On the Cure Research NewsProof-of-concept for RDH12 gene therapy demonstrated in mouse model
-
Jul 25, 2019
Allergan and Editas Begin Recruiting for CRISPR/Cas9 Clinical Trial for LCA10
Eye On the Cure Research NewsThe trial will be the first for a CRISPR/Cas9 therapy administered inside the human body.
-
Jun 17, 2019
The Retina is a Proving Ground for a Broad Range of Neurological Therapies
Science EducationRetinal research paves the way for new treatments for the entire neurological system.
-

May 20, 2019
Dr. Don Zack Honored for Research Contributions by ARVO and the Foundation Fighting Blindness
Eye On the Cure Research NewsDr. Zack is a member of the Foundation’s Scientific Advisory Board and chairs its Cellular Molecular Mechanisms of Disease study section.
-

May 9, 2019
Eye Bonds Re-Introduced to New Congress: Potentially $1 Billion in Government-Backed Funding for Eye Research
Eye On the Cure Research NewsEye Bonds provide the opportunity to advance, and accelerate development for, more promising treatments into and through clinical trials and out to the people who need them.
-

Apr 16, 2019
First Patient Receives AON Therapy for LCA10 in ProQR’s Phase 2/3 Clinical Trial
Eye On the Cure Research NewsThe treatment, formerly known as QR-110, is designed for people with the retinal disease Leber congenital amaurosis 10
-

Jan 29, 2019
The Foundation Receives a $100,000 Research Grant from Sofia Sees Hope
Eye On the Cure Research NewsSofia Sees Hope, a nonprofit dedicated to finding treatments and cures for people with Leber congenital amaurosis (LCA) and other inherited retinal diseases (IRDs), has made a $100,000 donation to the Foundation Fighting Blindness to support therapy development and genetic testing.
-
Jan 17, 2019
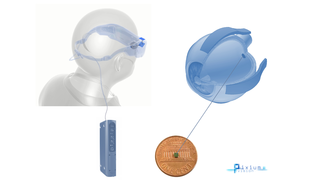
Pixium's PRIMA Bionic Vision System Restores Central Vision in Dry AMD Clinical Trial
Eye On the Cure Research NewsThe French bioelectronics company Pixium Vision has reported that its PRIMA bionic vision system has restored some central vision in patients with advanced dry age-related macular degeneration (AMD) participating in a clinical feasibility trial.
-

Dec 7, 2018
FDA Authorizes Clinical Trial for CRISPR/Cas9 Therapy for LCA 10
Eye On the Cure Research NewsEditas Medicine, a company developing gene-editing treatments, has received authorization from the US Food and Drug Administration to launch a clinical trial for its emerging CRISPR/Cas9 therapy for people with a mutation in the gene CEP290, which causes Leber congenital amaurosis 10 (LCA10). LCA causes severe vision loss or blindness at birth.
-

Nov 2, 2018
Foundation Invests $2.5 Million in Search for Elusive Retinal Disease Genes and Mutations
Eye On the Cure Research NewsSince 1989 genetic researchers, many funded by the Foundation, have identified approximately 270 genes linked to IRDs. In most cases, defects in a single gene can cause a retinal disease and vision loss.
-

Sep 11, 2018
FFB Congratulates RPE65 Gene Therapy Researchers for Champalimaud Award
Eye On the Cure Research NewsOn September 4, 2018, seven researchers, including six previously funded by the Foundation, were recognized with the prestigious 2018 Antonio Champalimaud Vision Award for their contributions to the advancement of blindness-reversing RPE65 gene therapies.
-

Sep 5, 2018
Vision Improvements Reported in ProQR's Clinical Trial for LCA10 Treatment
Eye On the Cure Research NewsThe company reported that 60 percent of subjects in the trial demonstrated improvements in visual acuity and their ability to navigate a mobility course. The treatment was also safe for patients.
-
Aug 15, 2018
FFB Provides Four Career Development Awards to Up-and-Coming Clinical Researchers
Eye On the Cure Research NewsEach recipient will receive a total of $375,000 over five years to help build an independent research program in addition to their clinical practices.
-
Aug 6, 2018
FFB Funding More than $2 Million in New Research
Eye On the Cure Research NewsSeventy scientists submitted requests for funding.
-

Jul 20, 2018
Call to Action: Ask Congress to Support $1 Billion in Eye Research
Eye On the Cure Research NewsCall to Action: Ask Congress to Support $1 Billion in Eye Research
-
Jul 5, 2018
Retinal Regeneration: Releasing Your Inner Salamander
Eye On the Cure Research NewsMany research groups from around the world are investigating ways to create new photoreceptors from stem cells for transplantation into the retina for vision restoration.
-
Jun 22, 2018
VISIONS2018 Live Stream
Eye On the Cure Research NewsWatch recorded sessions from VISIONS2018.
-

May 30, 2018
French Gene Therapy Company Advancing Three Programs for Retinal Diseases
Eye On the Cure Research NewsHorama’s gene therapies are injections of healthy copies of a gene underneath the retina to compensate for the defective gene.
-

May 3, 2018
ARVO 2018: Dr. Stephen Daiger Reports on the State of Genetic Testing for Inherited Retinal Diseases
Eye On the Cure Research NewsARVO 2018: Dr. Stephen Daiger Reports on the State of Genetic Testing for Inherited Retinal Diseases
-

May 2, 2018
ARVO 2018: Dr. Steve Rose Reports on CRISPR/Cas9 for Inherited Retinal Diseases
Eye On the Cure Research NewsFFB’s own Dr. Steve Rose, chief scientific officer, reviews our commitment to funding and exploring CRISPR/Cas9 gene editing for inherited retinal disease.
-

Apr 30, 2018
ARVO 2018: Dr. Shannon Boye Reports on her Emerging Gene Therapy for LCA (GUCY2D)
Eye On the Cure Research NewsShannon Boye, PhD, University of Florida, talks about the advancement of her of gene therapy for Leber congenital amaurosis toward a clinical trial.
-

Apr 25, 2018
ARVO 2018: World's Largest Show and Tell for Innovations in Eye Research
Eye On the Cure Research NewsMore than 11,000 eye researchers from around the world — including five intrepid members from FFB’s science team — will gather to participate in what is essentially a massive “show and tell” of the latest scientific advancements.
-
Mar 7, 2018
Natural History Study Launches for LCA Caused by Specific Mutation in CEP290
Eye On the Cure Research NewsThe natural history study will be used to characterize the range of visual function in patients, to evaluate which visual tests may be the most useful for patients with this condition, and to determine the rate of change in visual function over a one-year period.
-
Jan 17, 2018
Clinical Trial to Launch for System Combining Optogenetics and Eyewear
Eye On the Cure Research NewsThe French biotech GenSight Biologics has received regulatory authorization in the UK to launch the PIONEER Phase 1 \ 2 clinical trial for its GS030 system — a light-sensing gene therapy (optogenetics) coupled with eyewear, which enhances visual stimulation.
-

Jan 9, 2018
Top Retinal Research Advances for 2017
Eye On the Cure Research NewsAn exciting year in fighting blindness.
-
Dec 20, 2017
History Is Made: FDA Approves Spark's Vision-Restoring Gene Therapy
Eye On the Cure Research NewsKnown as LUXTURNA™ (voretigene neparvovec), the gene therapy restored vision in a clinical trial for people between the ages of 4 and 44 with Leber congenital amaurosis (LCA) caused by mutations in the gene RPE65.
-
Nov 21, 2017
Stem-Cell Therapy Clinics Remain Inadequately Regulated, Pose Risk to Patients
Eye On the Cure Research NewsIf a clinic is charging for a stem-cell treatment or procedure for an IRD, it is probably not legit. The expense to the patient is a major red flag.
-
Nov 15, 2017
ProQR Doses First Participant in Its LCA10 Therapy Clinical Trial
Eye On the Cure Research NewsThe company plans to report interim, six-month study results in 2018 and 12-month results in 2019.
-

Oct 13, 2017
FDA Committee Unanimously Recommends Approval for Spark's RPE65 Gene Therapy - Final Decision Due in January 2018
Eye On the Cure Research NewsAn advisory committee comprised of FDA-selected experts voted unanimously – 16 to 0 – to recommend approval.
-

Sep 27, 2017
The Foundation's Investments Are Filling the Pipeline for Vision-Saving Therapies
Eye On the Cure Research NewsIn addition to funding promising biotech start-ups, the Foundation Fighting Blindness has played a critical role in developing research talent.
-
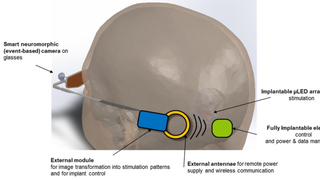
Jul 26, 2017
Scientists Receive $25 Million to Develop a Vision-Restoring System that Connects to the Brain
Eye On the Cure Research NewsThe high-tech, vision-restoring system interfaces with the visual cortex, the back of the brain where visual input is processed to create the images we see.
-
Jul 25, 2017
Foundation Fighting Blindness and 4D Molecular Therapeutics Partner to Boost Retinal Gene Therapy Development
Eye On the Cure Research NewsThe partnership will help companies and researchers quickly obtain and implement high-quality vectors for their retinal gene-therapy development efforts.
-
Jul 24, 2017
FFB-Funded Scientists Report on Nine Promising Translational Research Efforts
Eye On the Cure Research NewsThe Foundation Fighting Blindness has taken the translational challenge head on by investing more than $75 million in therapy-development projects with strong clinical-trial potential through its Translational Research Acceleration Program (TRAP), which includes Gund-Harrington Scholar Awards.
-

May 23, 2017
Forty-Four High-Impact Retinal-Research Efforts Highlighted at FFB-Casey Innovation Summit
Eye On the Cure Research NewsIn its fourth year, the meeting is becoming the world’s most comprehensive overview of the promising research underway for emerging IRD treatments.
-
May 16, 2017
Clinical Trial Authorized in the U.S. for Emerging LCA 10 Therapy
Eye On the Cure Research NewsProQR, a biotechnology company in the Netherlands, has received authorization from the U.S. Food and Drug Administration to start a Phase I/II clinical trial for its therapy known as QR-110
-

May 8, 2017
FFB Funding Helps Retinal Genetics Lab Secure $2 Million Investment
Eye On the Cure Research NewsHow the Foundation Fighting Blindness (FFB) provided timely funding of $155,000 to help a lab at the University of California, San Diego (UCSD), leverage a $2 million retinal-gene discovery project.
-

Mar 22, 2017
Dr. Eliot Berson, Pioneer in Vitamin A Therapy for Retinitis Pigmentosa, Passes Away
Eye On the Cure Research NewsDr. Berson dedicated himself to clinical care and vision-saving research for people with inherited retinal diseases for five decades.
-
Mar 16, 2017
Unregulated Stem-Cell Therapy Causes Severe Vision Loss for Three Florida Women
Eye On the Cure Research News“…participation in a study for an emerging therapy that is not regulated by the FDA or another well-recognized regulatory agency like the European Medicines Agency in Europe, is fraught with dangers and can lead to unexpected serious consequences.”
-
Feb 17, 2017
AGTC Leverages Funding from the Foundation to Move Promising Treatments into Clinical Trials
Eye On the Cure Research NewsCompany Builds on FFB’s Initial Investment to Garner $265 Million in Therapy Development Funding
-
Dec 21, 2016
FFB-CRI Leads Effort to Identify Outcome Measures for Therapies in Clinical Trials
Eye On the Cure Research NewsImproved outcome measures will make clinical trials for degenerative retinal diseases — including age-related macular degeneration (AMD), the world’s leading cause of blindness in seniors, and inherited retinal conditions such as RP and Stargardt disease — less expensive to conduct and able to deliver more precise results.
-
Oct 18, 2016
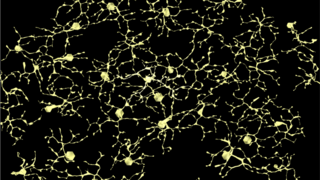
Building a Wiring Diagram for the Retina to Help Researchers Save and Restore Vision
Eye On the Cure Research NewsUnderstanding the pathways of the retinal neural network — and how they are rewired with aging and disease — is helpful in trying to save and restore vision.
-

Oct 11, 2016
Nobel-Prize-Winning Stem-Cell Researcher Delivers Keynote at FFB-Funded Conference in Kyoto
Eye On the Cure Research NewsDr. Shinya Yamanka discussed his early clinical trial for iPSC-derived retinal pigment epithelial (RPE) cells for a 78-year-old woman with advanced wet age-related macular degeneration (AMD).
-
.png)
Oct 6, 2016
Embrace Your Exceptions: A Mantra for Understanding Retinal-Disease Inheritance
Eye On the Cure Research NewsThe complex and elusive nature of these conditions can also extend to the way they are passed down in families, making diagnosis and prognosis quite challenging.
-

Sep 6, 2016
Researchers Identify Canine Model of LCA (NPHP5) — Pursue Gene Therapy
Eye On the Cure Research NewsThe investigators found that in canines, the retinal degeneration is remarkably similar to that in humans with NPHP5 mutations.
-

Aug 18, 2016
Optogenetic Therapy Takes First Step Forward in Clinical Trial
Eye On the Cure Research NewsRetroSense’s optogenetic therapy is designed to restore vision to people who are completely blind from retinal degenerative diseases such as retinitis pigmentosa by bestowing light sensitivity to retinal ganglion cells, which survive after photoreceptors, the cells that make vision possible, are lost.
-

Aug 2, 2016
Pixium Vision Reports Progress in Development of Two Advanced Bionic Retina Systems
Eye On the Cure Research NewsBoth approaches show strong, near-term potential for providing meaningful vision to people who are otherwise blind from retinal diseases such as retinitis pigmentosa and age-related macular degeneration (AMD).
-

Jul 1, 2016
VISIONS 2016 - Dr. Richard Weleber Receives FFB's Highest Research Honor, Recognized in Touching Video
Eye On the Cure Research NewsDr. Weleber became the 10th recipient of the Foundation’s highest honor, named after FFB co-founder Lulie Gund, during the opening lunch of the VISIONS 2016 conference.
-
Jul 1, 2016
VISIONS 2016 — Dr. Shomi Bhattacharya Wins FFB Award for Gaining an Understanding of Variations in Vision Loss
Eye On the Cure Research NewsAt VISIONS 2016, FFB’s national conference, the Foundation honored him with its Ed Gollob Board of Directors Award for breakthrough research conducted within the past year.
-
Jun 24, 2016
A Steady Hand in Saving Vision
Eye On the Cure Research NewsSubretinal injection is the most common form of delivery for gene therapies currently in clinical trials.
-

Oct 8, 2015
A Leap Forward: Spark Therapeutics Seeks FDA Approval for its Vision-Restoring Gene Therapy
Eye On the Cure Research News -
Jun 27, 2015
VISIONS 2015 — Dr. José Sahel Receives Foundation's Most Prestigious Research Honor
Eye On the Cure Research NewsFor those of us supporting the drive for vision-saving treatments and cures, he’s exactly the type of person we want on our team.
-

Jun 26, 2015
VISIONS 2015 — Dr. Shannon Boye Receives FFB Award for Excellence in Gene-Therapy Research
Eye On the Cure Research NewsDr. Boye received the Foundation’s Board of Director’s Award, which was presented at VISIONS 2015, FFB’s annual conference, for achievements in retinal research.
-
May 19, 2015
ARVO 2015 Highlight: The National Eye Institute Invests $4 Million in Audacious-Goals Research
Eye On the Cure Research NewsThe mission of the program—to regenerate the neurons and neural connections in the eye and visual system—is synonymous with the Foundation’s mission to eradicate retinal diseases.
-
May 12, 2015
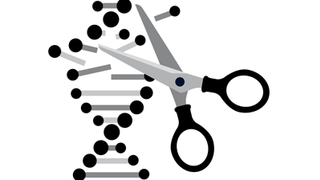
ARVO 2015 Highlight: A Cut-and-Paste Approach to Fixing Retinal-Disease Genes
Eye On the Cure Research NewsOne of the hot topics at ARVO this year is a rapidly advancing gene-therapy approach called clustered regularly interspaced short palindromic repeats, or CRISPR.
-

Aug 1, 2014
How Evolution is Leading to Gene Therapies for More Retinal Diseases
Eye On the Cure Research NewsAn innovative genetic-engineering approach called “directed evolution” to find optimal gene-delivery systems based on adeno-associated viruses (AAVs).
-
Jun 21, 2014
VISIONS 2014 — My Retina Tracker: Track Your Vision and Drive the Research
Eye On the Cure Research NewsThe powerful and secure system enables patients to keep track of their clinical care and vision changes. At the same time, it enables scientists to search the “de-identified” (i.e., anonymous) patient information to study conditions and identify targets for treatments, preventions and cures.
-
Jun 21, 2014
VISIONS 2014 — The Multi-Talented Dr. Shannon Boye
Eye On the Cure Research NewsDr. Boye and her research team received a $900,000 grant for a gene therapy project targeting Leber congenital amaurosis.
-
May 8, 2014
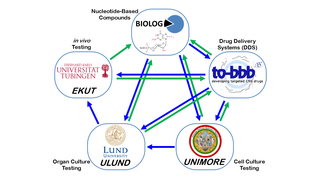
ARVO 2014: European Collaboration Developing Cross-Cutting, Vision-Saving Therapies
Eye On the Cure Research NewsSimply put, they’re creating therapies that can save vision in as many people as possible, independent of the genetic cause of disease.
-
Apr 8, 2014
Total Blindness and Non-24 Sleep Disorder
Eye On the Cure Research NewsNon-24 is a very rare condition affecting many (but not all) people who are totally blind and have absolutely no light perception. Their circadian clocks become out of sync as a result.
-
Dec 31, 2013
Nouvelle Lumière: French Bionic Retina in a Human Study
Eye On the Cure Research NewsThe French retinal implant developer Pixium quietly launched a clinical trial for its Intelligent Retinal Implant System 1 (IRIS1) in France, Austria and Germany.
-
Aug 9, 2013
When a Condition is More than a Retinal Disease
Science EducationThe Foundation Fighting Blindness is, of course, all about finding treatments and cures for retinal degenerative diseases. However, we are well aware that many of our constituents and their families are dealing with more than just vision loss. That’s because genetic defects causing retinal conditions can sometimes affect other parts of the body. The result is conditions often referred to as syndromes.
-
Jul 26, 2013
Researchers Move Closer to Getting a Complete Genetic Picture of the Retina
Eye On the Cure Research NewsIdentifying the genes and proteins that play a major role in retinal health and vision is an important step in finding preventions and cures for degenerative diseases.
-
Jun 12, 2013
Patient Registries Help Advance Research for Rare Diseases
Eye On the Cure Research NewsMany registries enable patients to collect and track information about their health, so they can take an active role in managing their care.
-
May 10, 2013
Grow Your Own: Harnessing Muller Glia for Retinal Regeneration
Eye On the Cure Research NewsThere’s hope for retinal regeneration for humans, thanks to Foundation-funded researcher Dr. Thomas Reh, who is investigating how to derive new photoreceptors from retinal cells called Muller glia.
-
May 7, 2013
Retinal Regeneration is Major Focus of NEI's Audacious Goal
Eye On the Cure Research NewsThe goal, “to regenerate the neurons and neural connections in the eye and visual system,” is exactly what people with retinal diseases need to save and restore their vision.
-

Apr 30, 2013
Researcher Revolutionized Fight Against Blindness and Cancer
Eye On the Cure Research NewsA profile on Dr. Robert Langer, a medical researcher who has received dozens of awards, accolades and honorary degrees, including, recently, FFB’s Visionary Award.
-
Mar 8, 2013
Staying Alive: Saving Retinal Cells to Preserve Vision
Science EducationSometimes, saving vision simply comes down to keeping retinal cells alive, or at least slowing their degeneration.
-

Feb 18, 2013
History in the Making
Eye On the Cure Research NewsMore good news about treatments and technological advances for restoring vision for people with retinal diseases.
-
Jun 19, 2012
Have I Got a Cure for You! Debunking an Alleged Treatment on the Internet
Eye On the Cure Research NewsHow do you know if a treatment is legit? There should be preclinical and clinical trial data published in a peer-reviewed journal on research for the treatment.
Related Resources
-

Apr 11, 2022
“The Only Disability is a Bad Attitude”
Beacon StoriesBorn with LCA, Anthony began wrestling in the seventh grade, continued through high school, and eventually became the subject of a documentary about his life as a blind wrestler. He then received a life-changing call from the United States Olympics Committee, asking him to compete on the Paralympic Judo Team.
-

Nov 15, 2021
Livie’s “New Eyes”
Beacon StoriesAt the age of five, Livie was diagnosed with LCA but her doctor immediately told her parents that clinical trials were underway and that Livie could soon be eligible for treatment. Not long after, they got a phone call from Spark Therapeutics and Livie received LUXTURNA®. Now eight, Livie’s confidence is at an all-time high with her “new eyes.”
-

Dec 21, 2020
Hannah’s Bright Future Ahead
Beacon StoriesAt seven years old, Hannah had never seen a lightning bug in the summer or a star in the sky. But after her treatment with LUXTURNA, Hannah can now play outside at night with her brothers and has a new bright future in store.
-

Sep 25, 2020
ProQR Announces Virtual Presentations at Scientific Conferences
ResourceProQR Therapeutics N.V. (Nasdaq:PRQR), a company dedicated to changing lives through the creation of transformative RNA therapies for severe genetic rare diseases, today announced virtual presentations at the Ophthalmology Futures Retina Forum, European Society of Retina Specialists (Euretina) congress and the Annual Meeting of the American Academy of Optometry (AAOpt).
-

Mar 9, 2020
No Slowing Down for LCA
Beacon StoriesBraydon was diagnosed with an inherited retinal disease at only two years old. Eight years later, after his mom enrolled him in the My Retina Tracker® Program, Braydon learned his disease was LCA.
-

Dec 17, 2019
Ashlyn Experiences Joy in Day-To-Day Life After Sight Is Restored
Beacon StoriesAshlyn’s vision was restored following her treatment with the LUXTURNA gene therapy.
-

Jul 22, 2019
Kailey’s Story of Hope
Beacon Stories13 year old Kailey Reichardt’s personal essay about her little sister Ashlyn, who was diagnosed with Leber congenital amarosis (LCA) at a young age. Kailey is a Beacon for other siblings impacted and going through similar situations.
-

Oct 16, 2018
A Boy with No Boundaries
Beacon StoriesKai Wang was 18 months old when he was diagnosed with the condition. His parents never imagined the extraordinary journey they would take with their son when they learned he had a condition that would render him blind.
-

Aug 7, 2017
Sight and Song: The Christian Guardino Story
Beacon StoriesChristian Guardino has been singing for as long as he can remember; it’s the seeing that is something new.
-

Oct 14, 2014
Changing Someone’s Life: A New Video Emphasizes the Need to Support FFB’s Mission
Beacon StoriesAllison Corona is one of 40 people with Leber congenital amaurosis who’ve benefitted from a gene therapy clinical trial.
-
Dec 18, 2012
Curing Blindness, Part 1: Corey’s Story
Beacon StoriesHow gene therapy restored some of a boy’s sight.




