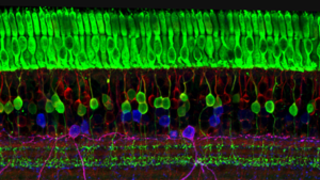
Other Retinal Conditions
Other Retinal Conditions
Batten disease
Also known as neuronal ceroid lipofuscinosis, refers to a group of rare inherited neurological conditions that can cause vision loss, progressive motor and cognitive decline, and seizures. There are different types of Batten disease, classified according to age at the onset of symptoms. Disease symptoms can be manifest at birth (congenital), or appear during childhood (infantile, late infantile, and juvenile) or adulthood. Childhood forms of Batten are the most common, often appearing between the ages of 5 and 10 as vision problems due to retinal degeneration or epilepsy (seizures) — or simply as problems with learning and general clumsiness.
Bietti crystalline dystrophy
Bietti crystalline dystrophy (BCD) is a rare autosomal recessive ocular disease that is characterized by yellow-white crystalline lipid deposits in the retina and sometimes cornea, degeneration of the retinal pigment epithelium (RPE), and sclerosis of the choroidal vessels. Progression of the disease ultimately results in reduced visual acuity, night blindness, visual field loss, and impaired color vision. Onset of the disease can occur from early teenage years to third decade of life, but can also occur beyond the third decade. As the disease progresses, decreases in peripheral acuity, central acuity or both ultimately results in legal blindness in most patients.
Blue-Cone Monochromacy
Blue cone monochromatism is characterized by poor central vision and color discrimination, infantile nystagmus, and nearly normal retinal appearance. The psychophysiologic functions of both rods and blue cones are preserved (Lewis et al., 1987). The frequency of achromatopsia is said to be approximately 1 in 100,000 persons. The first detailed description is that given by Huddart (1777). The subject of that report 'could never do more than guess the name of any color; yet he could distinguish white from black, or black from any light or bright color...He had 2 brothers in the same circumstances as to sight; and 2 brothers and sisters who, as well as his parents, had nothing of this defect.' This disorder was previously interpreted as total colorblindness. Information presented by Spivey (1965) indicated that affected persons can see small blue objects on a large yellow field and vice versa. These cases have been variously called partial complete colorblindness, or incomplete achromatopsia. Blackwell and Blackwell (1961) have described achromatopic families in which a few blue cones seemed to be present. See comments of Alpern et al. (1960). Sloan (1964) also had evidence of the presence of a few red cones in cases of otherwise complete achromatopsia. Bromley (1974) showed me a large kindred with this disorder in a typical X-linked recessive pattern.
Cohen syndrome
Cohen syndrome is a variable genetic disorder characterized by diminished muscle tone (hypotonia), abnormalities of the head, face, hands and feet, eye abnormalities, and non-progressive intellectual disability. Affected individuals usually have microcephaly, a condition in which head circumference is smaller than would be expected for an infant’s age and sex. In many older patients, obesity is present, especially around the torso and is associated with slender arms and legs. A lowered level of certain white blood cells known as neutrophils (neutropenia) is present from birth in some affected individuals. Cohen syndrome is an autosomal recessive genetic disease caused by mutations in the VPS13B/COH1 gene. Affected individuals may also have chorioretinal dystrophy, a condition characterized by abnormalities affecting the choroid and retina including degeneration of the retina.
Cone-Rod Dystrophy
Cone-rod retinal dystrophy (CRD) characteristically leads to early impairment of vision. An initial loss of color vision and of visual acuity is followed by nyctalopia (night blindness) and loss of peripheral visual fields. In extreme cases, these progressive symptoms are accompanied by widespread, advancing retinal pigmentation and chorioretinal atrophy of the central and peripheral retina (Moore, 1992). Evans et al. (1995) found complete blindness (no light perception) in only 3 of the 34 patients studied, and these 3 were all over 65 years of age. Serious effects on visual acuity (light perception only) were present in 10 other patients; however, their mean age was 60.3 years. All other patients retained some visual acuity. In many families, perhaps a majority, atrophy of the central and peripheral choreoretinal atrophy is not found (Tzekov, 1998).
The most common genes associated with cone-rod dystrophy are CNGA3, CNGB3, and RPGR.
Enhanced S-cone syndrome
Enhanced S-cone syndrome (ESCS) is an autosomal recessive retinopathy in which patients have increased sensitivity to blue light; perception of blue light is mediated by what is normally the least populous cone photoreceptor subtype, the S (short wavelength, blue) cones. People with ESCS also suffer vision loss, with night blindness occurring from early in life, varying degrees of L (long, red)- and M (middle, green)-cone vision, and retinal degeneration. The pattern of retinal dysfunction is a constant among ESCS patients, but the degree of clinically evident retinal degeneration can vary from minimal to severe. The condition is caused by mutations in the gene NR2E3.
Goldmann-Favre syndrome
Goldmann-Favre syndrome is the severe form of enhanced S-cone syndrome. It’s characterized by a liquefied vitreous body with preretinal band-shaped structures (veil), macular changes in the form of retinoschisis or edema, and pigmentary degeneration of the retina with problems with vision in bright light and extinguished electroretinogram. Cataracts are a complication.
Gyrate atrophy
Gyrate atrophy of the choroid and the retina is a rare autosomal recessive retinal dystrophy characterized by progressive chorioretinal degeneration, early cataract formation, and myopia. It is caused by a deficiency in the enzyme ornithine aminotransferase (OAT), which results in a 10- to 20-fold increase in plasma ornithine concentrations. Patients classically present in the first decade of life with night blindness, with fundus exam revealing characteristic circular patches of chorioretinal atrophy distributed in the peripheral fundus. As the disease progresses, the atrophic lesions coalesce and advance centripetally toward the posterior pole, correlating with progressive loss of peripheral vision. Macular involvement occurs late in the disease.
Heimler syndrome
Heimler syndrome (HS) is characterized by sensorineural hearing loss, tooth enamel hypoplasia, nail abnormalities, and occasional or late-onset retinal degeneration (macular dystrophy). HS-causing mutations in the PEX1 and PEX6 genes represent the mild end of the Zellweger syndrome spectrum disorders (ZSSD).
Joubert syndrome
Joubert syndrome is a rare genetic disorder in infants and children whose brains don’t develop correctly. A part of the brain called the cerebellar vermis, which controls balance and coordination, is either underdeveloped or absent. And the brain stem, which connects the brain and spinal cord, is also abnormal. Joubert syndrome can affect many different parts of the body. It can lead to multiple health problems, developmental delays, intellectual disability, and retinal degeneration.
Malattia Leventinese
Characteristically small round white spots (drusen) involving the posterior pole of the eye, including the areas of the macula and optic disc, appear in early adult life. Progression to form a mosaic pattern which Doyne (1899) aptly termed 'honeycomb' occurs thereafter. Doyne considered it to represent 'choroiditis.' However, Collins (1913) showed that the changes consisted of swelling in the inner part of Bruch membrane. Failing vision usually developed considerably later than the ophthalmologic change. Robert Walter Doyne (1857-1916) was an ophthalmologist in Oxford, England. Pearce (1967) did an extensive study of 6 kindreds living near Oxford. Some and possibly all may have been descendants from a common ancestor. Dominant inheritance with complete manifestation of the trait in persons surviving beyond early adult life was found. Families living elsewhere than England have been reported (see references given by Pearce, 1968). Maumenee (1982) suggested that this may be fundamentally the same disorder as drusen of Bruch membrane (126700).
North Carolina macular dystrophy
North Carolina macular dystrophy (NCMD) is a non-progressive autosomal dominant macular disorder of congenital or infantile onset characterized by loss of central vision, the accumulation of drusen in the macula and atrophy of photoreceptor cells with a variable phenotype at macular examination. The severity of changes in the development of the macula varies, causing some people to have little or no vision loss, while others may have severe vision loss.
Oguchi Disease
The characteristics are congenital, static hemeralopia and diffuse yellow or gray coloration of the fundus. After 2 or 3 hours in total darkness, the normal color of the fundus returns. The condition is more frequent in Japanese. See hemeralopia (310500) for a comment on the use of this term, as opposed to the term nyctalopia.
Pattern dystrophies
Pattern dystrophies are a group of autosomal dominant macular diseases characterized by various patterns of pigment deposition within the macula. The primary layer of the retina effected is the retinal pigment epithelium (RPE) which is responsible for removing and recycling waste within the retina. In various pattern dystrophies, these wastes accumulate in the form of lipofuscin. Pattern dystrophies are often associated with a relatively good visual prognosis, although slow progressive central vision loss can occur. Disease onset typically occurs in patients during their forties and fifties. However, older patients may be misdiagnosed as age-related macular degeneration due to similar macular patterns of pigment deposition.
Refsum disease
Refsum disease is an extremely rare and complex disorder that affects many parts of the body. A form of the retinal degenerative disease known as retinitis pigmentosa (RP) is a common feature of this disease.
Retinitis punctata albescens
Retinitis punctata albescens is a progressive form of autosomal recessive flecked retinopathy characterized by white punctata throughout the fundus (but sparing the macula in the early stages). Patients present with night blindness in childhood and may also experience a loss of visual acuity. Significant loss of vision is reported in the 5th and 6th decades of life.
Rod-Cone Dystrophy
Rod-cone dystrophy results from a primary loss of rod photoreceptors, followed by loss of cones.
Senior-Loken syndrome
Senior-Løken syndrome is a rare disorder characterized by the combination of two specific features: a kidney condition called nephronophthisis and the retinal condition Leber congenital amaurosis. SLS is inherited in an autosomal recessive pattern. Currently, 10 genes have been linked to the disorder.
Sorsby fundus dystrophy
Sorsby fundus dystrophy (SFD) is an inherited, macular degenerative disorder caused by mutations in the Tissue Inhibitor of Metalloproteinase-3 (TIMP3) gene. SFD closely resembles age-related macular degeneration (AMD), which is the leading cause of blindness in the elderly population of the Western hemisphere. Variants in TIMP3 gene have recently been identified in patients with AMD. Patients with this condition typically maintain normal acuity until their 40s when they develop severe, bilateral choroidal neovascular membranes.
Zellweger syndrome
Zellweger syndrome is one of a group of four related diseases called peroxisome biogenesis disorders (PBD). The diseases are caused by defects in any one of 13 genes, termed PEX genes, required for the normal formation and function of peroxisomes. Peroxisomes are cell structures that break down toxic substances and synthesize lipids (fatty acids. oils, and waxes) that are necessary for cell function. Peroxisomes are required for normal brain development and function and the formation of myelin, the whitish substance that coats nerve fibers. People with these disorders can have retinal degeneration.
To learn about clinical trials underway for retinal diseases, visit www.ClinicalTrials.gov.
Next Section
Latest News
-

Mar 25, 2024
BlueRock Therapeutics and Foundation Fighting Blindness announce collaboration to expand the Uni-Rare natural history study of patients living with inherited retinal diseases
Press ReleasesCollaboration will add a new multi-gene cohort of patients living with inherited retinal diseases. Data insights from the new study cohort will inform the future clinical trial design for BlueRock’s pipeline of cell therapies for treating blindness.
-

Feb 6, 2024
Foundation Fighting Blindness Launches GYROS, a Natural History Study for People with Gyrate Atrophy
Press ReleasesGyrate atrophy is an inherited retinal disease—causing progressive vision loss. GYROS results will help researchers design clinical trials for an emerging gyrate atrophy gene therapy.
-

Aug 4, 2020
Foundation Insights Forum – July 30, 2020
Insights ForumThe Foundation Fighting Blindness is pleased to provide an audio recording and full transcript of the Insights Forum, our quarterly conference call providing updates to the inherited retinal disease community. The call took place on July 30, 2020.
-
Mar 31, 2020
COVID-19 Resources
Foundation NewsThe Foundation Fighting Blindness is closely monitoring the COVID-19 situation and its impact on the IRD community.
-
Feb 6, 2020
Foundation Insights Forum – January 31, 2020
Insights ForumThe Foundation Fighting Blindness is pleased to provide an audio recording and full transcripts of the Insights Forum, our quarterly conference call providing updates to the inherited retinal disease community. The call took place on January 31, 2020.
-

Feb 6, 2020
ProQR Therapeutics Teams Up with the Foundation Fighting Blindness and Blueprint Genetics to Support the My Retina Tracker® Program for People Living with Inherited Retinal Diseases
Press ReleasesMy Retina Tracker Program is the highest volume IRD genetic testing program in the U.S.
-
Nov 8, 2019
Foundation Insights Forum – October 30, 2019
Insights ForumThe Foundation Fighting Blindness is pleased to provide an audio recording and full transcript of the Insights Forum, our quarterly conference call providing updates to the inherited retinal disease community. The call took place on October 30, 2019.
-
Oct 2, 2019
Blueprint Genetics, InformedDNA and the Foundation Fighting Blindness launch an open access program for patients with inherited retinal disease in the United States
Press ReleasesThe program will offer patients with inherited retinal disease no-cost genetic testing and genetic counseling in the United States. Look for updated information on how to participate to be posted in mid-October, with program registration starting shortly thereafter.
-
Aug 16, 2019
Foundation Fighting Blindness Investing Nearly $6.5 Million in New Grants
Foundation NewsThe newly funded research efforts include several therapies that have strong potential to treat a wide range of inherited retinal diseases.
-

May 9, 2019
Foundation Fighting Blindness Endorses 'Eye Bonds' Legislation
Press ReleasesBipartisan Bill Will Stimulate Up to $1 Billion in New Funding for Blindness Research
-

Jul 19, 2018
Foundation Fighting Blindness Urges Congress to Pass ‘Eye-Bonds’ Legislation
Press ReleasesBill Introduced in U.S. House Would Speed Up Cures for Blindness
-

Jun 8, 2018
Foundation Fighting Blindness and CheckedUp® Partner to Educate Retinal-Disease Patients About Research, Resources, and Emerging Therapies During Doctor Visits
Press ReleasesThe Foundation Fighting Blindness (the Foundation) and CheckedUp have formed a collaborative partnership to deliver patient-friendly diagnostic and disease-management information to people with retinal diseases such as age-related macular degeneration, retinitis pigmentosa, and Stargardt disease during their visits to eye doctors.
Latest Research
-

Aug 22, 2023
FDA Approves 8 MG Dosing of Eylea for Wet AMD, Diabetic Macular Edema, and Diabetic Retinopathy
Eye On the Cure Research NewsNew, higher treatment dose reduces frequency of eye injections for patients
-

Feb 7, 2020
Genetic Testing for Inherited Retinal Diseases through the Foundation’s Open Access Program
Science EducationThe benefits of genetic testing for IRD patients, how to participate in the Foundation’s Open Access program, and what to expect from the genetic testing process.
-
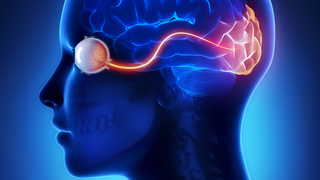
Jun 17, 2019
The Retina is a Proving Ground for a Broad Range of Neurological Therapies
Science EducationRetinal research paves the way for new treatments for the entire neurological system.
-

May 20, 2019
Dr. Don Zack Honored for Research Contributions by ARVO and the Foundation Fighting Blindness
Eye On the Cure Research NewsDr. Zack is a member of the Foundation’s Scientific Advisory Board and chairs its Cellular Molecular Mechanisms of Disease study section.
-

May 9, 2019
Eye Bonds Re-Introduced to New Congress: Potentially $1 Billion in Government-Backed Funding for Eye Research
Eye On the Cure Research NewsEye Bonds provide the opportunity to advance, and accelerate development for, more promising treatments into and through clinical trials and out to the people who need them.
-

Jan 29, 2019
The Foundation Receives a $100,000 Research Grant from Sofia Sees Hope
Eye On the Cure Research NewsSofia Sees Hope, a nonprofit dedicated to finding treatments and cures for people with Leber congenital amaurosis (LCA) and other inherited retinal diseases (IRDs), has made a $100,000 donation to the Foundation Fighting Blindness to support therapy development and genetic testing.
-
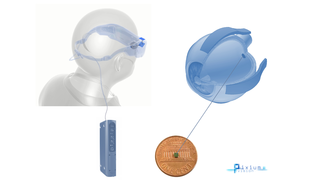
Jan 17, 2019
Pixium's PRIMA Bionic Vision System Restores Central Vision in Dry AMD Clinical Trial
Eye On the Cure Research NewsThe French bioelectronics company Pixium Vision has reported that its PRIMA bionic vision system has restored some central vision in patients with advanced dry age-related macular degeneration (AMD) participating in a clinical feasibility trial.
-

Nov 2, 2018
Foundation Invests $2.5 Million in Search for Elusive Retinal Disease Genes and Mutations
Eye On the Cure Research NewsSince 1989 genetic researchers, many funded by the Foundation, have identified approximately 270 genes linked to IRDs. In most cases, defects in a single gene can cause a retinal disease and vision loss.
-

Sep 11, 2018
FFB Congratulates RPE65 Gene Therapy Researchers for Champalimaud Award
Eye On the Cure Research NewsOn September 4, 2018, seven researchers, including six previously funded by the Foundation, were recognized with the prestigious 2018 Antonio Champalimaud Vision Award for their contributions to the advancement of blindness-reversing RPE65 gene therapies.
-
Aug 15, 2018
FFB Provides Four Career Development Awards to Up-and-Coming Clinical Researchers
Eye On the Cure Research NewsEach recipient will receive a total of $375,000 over five years to help build an independent research program in addition to their clinical practices.
-
Aug 6, 2018
FFB Funding More than $2 Million in New Research
Eye On the Cure Research NewsSeventy scientists submitted requests for funding.
-

Jul 20, 2018
Call to Action: Ask Congress to Support $1 Billion in Eye Research
Eye On the Cure Research NewsCall to Action: Ask Congress to Support $1 Billion in Eye Research
-
Jul 5, 2018
Retinal Regeneration: Releasing Your Inner Salamander
Eye On the Cure Research NewsMany research groups from around the world are investigating ways to create new photoreceptors from stem cells for transplantation into the retina for vision restoration.
-
Jun 22, 2018
VISIONS2018 Live Stream
Eye On the Cure Research NewsWatch recorded sessions from VISIONS2018.
-

May 3, 2018
ARVO 2018: Dr. Stephen Daiger Reports on the State of Genetic Testing for Inherited Retinal Diseases
Eye On the Cure Research NewsARVO 2018: Dr. Stephen Daiger Reports on the State of Genetic Testing for Inherited Retinal Diseases
-

May 2, 2018
ARVO 2018: Dr. Steve Rose Reports on CRISPR/Cas9 for Inherited Retinal Diseases
Eye On the Cure Research NewsFFB’s own Dr. Steve Rose, chief scientific officer, reviews our commitment to funding and exploring CRISPR/Cas9 gene editing for inherited retinal disease.
-

Apr 25, 2018
ARVO 2018: World's Largest Show and Tell for Innovations in Eye Research
Eye On the Cure Research NewsMore than 11,000 eye researchers from around the world — including five intrepid members from FFB’s science team — will gather to participate in what is essentially a massive “show and tell” of the latest scientific advancements.
-
Jan 17, 2018
Clinical Trial to Launch for System Combining Optogenetics and Eyewear
Eye On the Cure Research NewsThe French biotech GenSight Biologics has received regulatory authorization in the UK to launch the PIONEER Phase 1 \ 2 clinical trial for its GS030 system — a light-sensing gene therapy (optogenetics) coupled with eyewear, which enhances visual stimulation.
-

Jan 9, 2018
Top Retinal Research Advances for 2017
Eye On the Cure Research NewsAn exciting year in fighting blindness.
-
Dec 20, 2017
History Is Made: FDA Approves Spark's Vision-Restoring Gene Therapy
Eye On the Cure Research NewsKnown as LUXTURNA™ (voretigene neparvovec), the gene therapy restored vision in a clinical trial for people between the ages of 4 and 44 with Leber congenital amaurosis (LCA) caused by mutations in the gene RPE65.
-
Nov 21, 2017
Stem-Cell Therapy Clinics Remain Inadequately Regulated, Pose Risk to Patients
Eye On the Cure Research NewsIf a clinic is charging for a stem-cell treatment or procedure for an IRD, it is probably not legit. The expense to the patient is a major red flag.
-

Oct 13, 2017
FDA Committee Unanimously Recommends Approval for Spark's RPE65 Gene Therapy - Final Decision Due in January 2018
Eye On the Cure Research NewsAn advisory committee comprised of FDA-selected experts voted unanimously – 16 to 0 – to recommend approval.
-

Sep 27, 2017
The Foundation's Investments Are Filling the Pipeline for Vision-Saving Therapies
Eye On the Cure Research NewsIn addition to funding promising biotech start-ups, the Foundation Fighting Blindness has played a critical role in developing research talent.
-
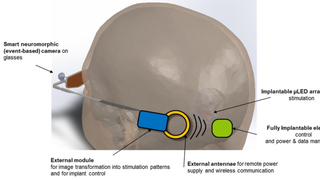
Jul 26, 2017
Scientists Receive $25 Million to Develop a Vision-Restoring System that Connects to the Brain
Eye On the Cure Research NewsThe high-tech, vision-restoring system interfaces with the visual cortex, the back of the brain where visual input is processed to create the images we see.
-
Jul 25, 2017
Foundation Fighting Blindness and 4D Molecular Therapeutics Partner to Boost Retinal Gene Therapy Development
Eye On the Cure Research NewsThe partnership will help companies and researchers quickly obtain and implement high-quality vectors for their retinal gene-therapy development efforts.
-
Jul 24, 2017
FFB-Funded Scientists Report on Nine Promising Translational Research Efforts
Eye On the Cure Research NewsThe Foundation Fighting Blindness has taken the translational challenge head on by investing more than $75 million in therapy-development projects with strong clinical-trial potential through its Translational Research Acceleration Program (TRAP), which includes Gund-Harrington Scholar Awards.
-

May 23, 2017
Forty-Four High-Impact Retinal-Research Efforts Highlighted at FFB-Casey Innovation Summit
Eye On the Cure Research NewsIn its fourth year, the meeting is becoming the world’s most comprehensive overview of the promising research underway for emerging IRD treatments.
-

May 8, 2017
FFB Funding Helps Retinal Genetics Lab Secure $2 Million Investment
Eye On the Cure Research NewsHow the Foundation Fighting Blindness (FFB) provided timely funding of $155,000 to help a lab at the University of California, San Diego (UCSD), leverage a $2 million retinal-gene discovery project.
-

Mar 22, 2017
Dr. Eliot Berson, Pioneer in Vitamin A Therapy for Retinitis Pigmentosa, Passes Away
Eye On the Cure Research NewsDr. Berson dedicated himself to clinical care and vision-saving research for people with inherited retinal diseases for five decades.
-
Mar 16, 2017
Unregulated Stem-Cell Therapy Causes Severe Vision Loss for Three Florida Women
Eye On the Cure Research News“…participation in a study for an emerging therapy that is not regulated by the FDA or another well-recognized regulatory agency like the European Medicines Agency in Europe, is fraught with dangers and can lead to unexpected serious consequences.”
-
Feb 17, 2017
AGTC Leverages Funding from the Foundation to Move Promising Treatments into Clinical Trials
Eye On the Cure Research NewsCompany Builds on FFB’s Initial Investment to Garner $265 Million in Therapy Development Funding
-
Dec 21, 2016
FFB-CRI Leads Effort to Identify Outcome Measures for Therapies in Clinical Trials
Eye On the Cure Research NewsImproved outcome measures will make clinical trials for degenerative retinal diseases — including age-related macular degeneration (AMD), the world’s leading cause of blindness in seniors, and inherited retinal conditions such as RP and Stargardt disease — less expensive to conduct and able to deliver more precise results.
-
Oct 18, 2016
Building a Wiring Diagram for the Retina to Help Researchers Save and Restore Vision
Eye On the Cure Research NewsUnderstanding the pathways of the retinal neural network — and how they are rewired with aging and disease — is helpful in trying to save and restore vision.
-

Oct 11, 2016
Nobel-Prize-Winning Stem-Cell Researcher Delivers Keynote at FFB-Funded Conference in Kyoto
Eye On the Cure Research NewsDr. Shinya Yamanka discussed his early clinical trial for iPSC-derived retinal pigment epithelial (RPE) cells for a 78-year-old woman with advanced wet age-related macular degeneration (AMD).
-
.png)
Oct 6, 2016
Embrace Your Exceptions: A Mantra for Understanding Retinal-Disease Inheritance
Eye On the Cure Research NewsThe complex and elusive nature of these conditions can also extend to the way they are passed down in families, making diagnosis and prognosis quite challenging.
-

Aug 18, 2016
Optogenetic Therapy Takes First Step Forward in Clinical Trial
Eye On the Cure Research NewsRetroSense’s optogenetic therapy is designed to restore vision to people who are completely blind from retinal degenerative diseases such as retinitis pigmentosa by bestowing light sensitivity to retinal ganglion cells, which survive after photoreceptors, the cells that make vision possible, are lost.
-

Aug 2, 2016
Pixium Vision Reports Progress in Development of Two Advanced Bionic Retina Systems
Eye On the Cure Research NewsBoth approaches show strong, near-term potential for providing meaningful vision to people who are otherwise blind from retinal diseases such as retinitis pigmentosa and age-related macular degeneration (AMD).
-
Jul 1, 2016
VISIONS 2016 — Dr. Shomi Bhattacharya Wins FFB Award for Gaining an Understanding of Variations in Vision Loss
Eye On the Cure Research NewsAt VISIONS 2016, FFB’s national conference, the Foundation honored him with its Ed Gollob Board of Directors Award for breakthrough research conducted within the past year.
-

Jul 1, 2016
VISIONS 2016 - Dr. Richard Weleber Receives FFB's Highest Research Honor, Recognized in Touching Video
Eye On the Cure Research NewsDr. Weleber became the 10th recipient of the Foundation’s highest honor, named after FFB co-founder Lulie Gund, during the opening lunch of the VISIONS 2016 conference.
-
Jun 24, 2016
A Steady Hand in Saving Vision
Eye On the Cure Research NewsSubretinal injection is the most common form of delivery for gene therapies currently in clinical trials.
-

Oct 8, 2015
A Leap Forward: Spark Therapeutics Seeks FDA Approval for its Vision-Restoring Gene Therapy
Eye On the Cure Research News -
Jun 27, 2015
VISIONS 2015 — Dr. José Sahel Receives Foundation's Most Prestigious Research Honor
Eye On the Cure Research NewsFor those of us supporting the drive for vision-saving treatments and cures, he’s exactly the type of person we want on our team.
-

Jun 26, 2015
VISIONS 2015 — Dr. Shannon Boye Receives FFB Award for Excellence in Gene-Therapy Research
Eye On the Cure Research NewsDr. Boye received the Foundation’s Board of Director’s Award, which was presented at VISIONS 2015, FFB’s annual conference, for achievements in retinal research.
-
May 19, 2015
ARVO 2015 Highlight: The National Eye Institute Invests $4 Million in Audacious-Goals Research
Eye On the Cure Research NewsThe mission of the program—to regenerate the neurons and neural connections in the eye and visual system—is synonymous with the Foundation’s mission to eradicate retinal diseases.
-
May 12, 2015
ARVO 2015 Highlight: A Cut-and-Paste Approach to Fixing Retinal-Disease Genes
Eye On the Cure Research NewsOne of the hot topics at ARVO this year is a rapidly advancing gene-therapy approach called clustered regularly interspaced short palindromic repeats, or CRISPR.
-

Aug 1, 2014
How Evolution is Leading to Gene Therapies for More Retinal Diseases
Eye On the Cure Research NewsAn innovative genetic-engineering approach called “directed evolution” to find optimal gene-delivery systems based on adeno-associated viruses (AAVs).
-
Jun 21, 2014
VISIONS 2014 — My Retina Tracker: Track Your Vision and Drive the Research
Eye On the Cure Research NewsThe powerful and secure system enables patients to keep track of their clinical care and vision changes. At the same time, it enables scientists to search the “de-identified” (i.e., anonymous) patient information to study conditions and identify targets for treatments, preventions and cures.
-
Jun 21, 2014
VISIONS 2014 — The Multi-Talented Dr. Shannon Boye
Eye On the Cure Research NewsDr. Boye and her research team received a $900,000 grant for a gene therapy project targeting Leber congenital amaurosis.
-
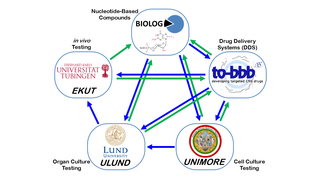
May 8, 2014
ARVO 2014: European Collaboration Developing Cross-Cutting, Vision-Saving Therapies
Eye On the Cure Research NewsSimply put, they’re creating therapies that can save vision in as many people as possible, independent of the genetic cause of disease.
-
Apr 8, 2014
Total Blindness and Non-24 Sleep Disorder
Eye On the Cure Research NewsNon-24 is a very rare condition affecting many (but not all) people who are totally blind and have absolutely no light perception. Their circadian clocks become out of sync as a result.
-
Dec 31, 2013
Nouvelle Lumière: French Bionic Retina in a Human Study
Eye On the Cure Research NewsThe French retinal implant developer Pixium quietly launched a clinical trial for its Intelligent Retinal Implant System 1 (IRIS1) in France, Austria and Germany.
-
Jul 26, 2013
Researchers Move Closer to Getting a Complete Genetic Picture of the Retina
Eye On the Cure Research NewsIdentifying the genes and proteins that play a major role in retinal health and vision is an important step in finding preventions and cures for degenerative diseases.
-
Jun 12, 2013
Patient Registries Help Advance Research for Rare Diseases
Eye On the Cure Research NewsMany registries enable patients to collect and track information about their health, so they can take an active role in managing their care.
-
May 10, 2013
Grow Your Own: Harnessing Muller Glia for Retinal Regeneration
Eye On the Cure Research NewsThere’s hope for retinal regeneration for humans, thanks to Foundation-funded researcher Dr. Thomas Reh, who is investigating how to derive new photoreceptors from retinal cells called Muller glia.
-
May 7, 2013
Retinal Regeneration is Major Focus of NEI's Audacious Goal
Eye On the Cure Research NewsThe goal, “to regenerate the neurons and neural connections in the eye and visual system,” is exactly what people with retinal diseases need to save and restore their vision.
-

Apr 30, 2013
Researcher Revolutionized Fight Against Blindness and Cancer
Eye On the Cure Research NewsA profile on Dr. Robert Langer, a medical researcher who has received dozens of awards, accolades and honorary degrees, including, recently, FFB’s Visionary Award.
-
Mar 8, 2013
Staying Alive: Saving Retinal Cells to Preserve Vision
Science EducationSometimes, saving vision simply comes down to keeping retinal cells alive, or at least slowing their degeneration.
-

Feb 18, 2013
History in the Making
Eye On the Cure Research NewsMore good news about treatments and technological advances for restoring vision for people with retinal diseases.
-
Jun 19, 2012
Have I Got a Cure for You! Debunking an Alleged Treatment on the Internet
Eye On the Cure Research NewsHow do you know if a treatment is legit? There should be preclinical and clinical trial data published in a peer-reviewed journal on research for the treatment.
Related Resources

May 18, 2020
An Artist, First and Foremost
Allen has always wanted to be known as an artist, first and foremost. His photography hints at the ever-changing nature of people’s lives and their environment, much like his own progression with retinitis pigmentosa (RP).




