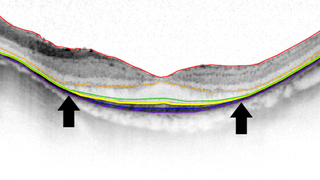
Often diagnosed in childhood or adolescence, retinitis pigmentosa (RP) is an inherited retinal disease causing progressive loss of night and peripheral vision. The condition often leads to legal and sometimes complete blindness.
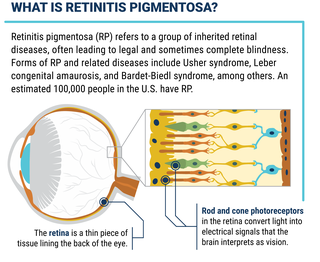
What is Retinitis Pigmentosa?
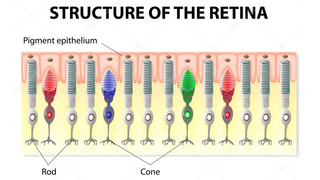
Retinitis pigmentosa, also known as RP, refers to a group of inherited diseases causing retinal degeneration and a decline in vision. The retina is a thin piece of tissue lining the back of the eye. Rod and cone photoreceptors in the retina convert light into electrical signals that the brain interprets as vision. People with RP experience a gradual decline in their vision, because photoreceptors degenerate.
Forms of RP and related diseases include Usher syndrome, Leber congenital amaurosis, and Bardet-Biedl syndrome, among others.
Symptoms
Symptoms depend on whether rods or cones are initially involved. In most forms of RP, rods are affected first. Because rods are concentrated in the outer portions of the retina and are activated by dim light, their degeneration affects peripheral and night vision. Vision becomes more constricted over time. If and when the disease progresses and cones become affected, visual acuity, color perception, and central vision are diminished.
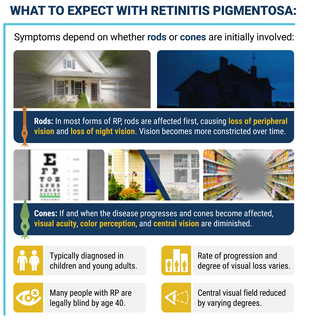
Night blindness is one of the earliest and most frequent symptoms of RP. People with mainly cone degeneration, however, first experience decreased central vision and reduced ability to discriminate colors and perceive details.
RP is typically diagnosed in children, adolescents and young adults. It is a progressive disorder. The rate of progression and degree of visual loss varies from person to person. Many people with RP are legally blind by age 40, with a central visual field of less than 20 degrees in diameter.
The most common genes associated with retinitis pigmentosa are PRPF31, PRPH2, RDH12, RHO, RPE65, ABCA4, MAK, MERTK, NR2E3, PDE6B, and RPGR.
Inheritance
An estimated 100,000 people in the U.S. have RP, mainly caused by mutations (variations) in a single gene inherited from one or both parents. The mutated gene gives the wrong instructions to photoreceptor cells, telling them to make an incorrect protein or too little or too much protein. (Cells need the proper amount of particular proteins in order to function properly.) Mutations in dozens of genes have been linked to RP.
Genetic mutations can be passed from parent to offspring through one of three genetic inheritance patterns — autosomal recessive, autosomal dominant, or X-linked.
In autosomal recessive RP, both parents carry one copy of the mutated gene and one normal copy, but have no symptoms themselves. They are therefore referred to as unaffected carriers. Each of their children has a 25 percent chance of being affected by inheriting a mutated copy from each parent. If the child inherits one mutated copy from one parent, they will be an unaffected carrier.
In autosomal dominant RP, usually one parent is affected and is the only parent with a mutated copy of the gene. A child has a 50 percent chance of being affected through the inheritance of the mutated copy from. The unaffected parent genetics doesn’t play a known role in the inheritance of the disease.
In X-linked RP, the mutated gene for the disease is located on the X chromosome. Females have two X chromosomes and can carry the disease gene on one of their X chromosomes. Because they have a healthy version of the gene on their other X chromosome, carrier females are less frequently affected by X-linked diseases. Males have only one X chromosome (paired with one Y chromosome) and are therefore genetically susceptible to X-linked diseases. Males with X-linked diseases pass their Y chromosome to their sons, and therefore will never pass an X-linked disease to their sons. Female carriers have a 50 percent chance (or 1 chance in 2) of passing the X-linked disease gene to their daughters, who become carriers, and a 50 percent chance of passing the gene to their sons, who are then affected by the disease.
If a family member is diagnosed with RP, it is often advised that other members of the family also have an eye exam by a physician who is specially trained to detect and treat retinal degenerative disorders. Genetic counselors are excellent resources for discussing inheritability, family planning, genetic testing, and other related issues.
Living with the Disease
There are many services and accommodative and assistive resources available to people and families with RP. Visit the Foundation’s Low Vision Resources page to learn about many of these resources. A low vision specialist can help recommend the resources that are right for you.
More information on managing RP is in the Newly Diagnosed section of this Web site.
Genetic Testing
Genetic testing is available for RP. It helps assess the risk of passing the disorder from parent to offspring. It also helps with attaining an accurate diagnosis. A patient with an accurate diagnosis is in a better position to understand which emerging treatment approaches and clinical trials are most appropriate for them.
Research and Clinical Trials
For the latest research advances for RP, refer to the Foundation publication Retinitis Pigmentosa: Research Advances.
View a list of current clinical trials, many made possible by Foundation support
RP page at www.ClinicalTrials.gov.

Join the fight and help us accelerate our mission
Next Section
Latest News
-

Mar 25, 2024
BlueRock Therapeutics and Foundation Fighting Blindness announce collaboration to expand the Uni-Rare natural history study of patients living with inherited retinal diseases
Press ReleasesCollaboration will add a new multi-gene cohort of patients living with inherited retinal diseases. Data insights from the new study cohort will inform the future clinical trial design for BlueRock’s pipeline of cell therapies for treating blindness.
-

Feb 23, 2024
Eye on the Cure Podcast | Episode 62: Dr. Peter Quinn
Foundation PodcastsPeter Quinn, PhD, a principal investigator and associate research scientist at Columbia University, talks to host Ben Shaberman about the promise of emerging CRISPR/Cas9 gene editing therapies, including base and prime editing approaches, for inherited retinal diseases. Dr. Quinn also reviews gene editing projects ongoing in his lab for patients with mutations in CRB1 and PRPH2.
-
.png)
Jan 20, 2023
Eye on the Cure Podcast – Episode 39: Paul Bernstein, MD, PhD
Foundation PodcastsJanuary 20, 2023. Paul Bernstein, MD, PhD, from the Moran Eye Center, University of Utah, and a member of the Foundation’s Scientific Advisory Board talks to host Ben Shaberman about his clinical practice for retinal disease patients
-

Nov 10, 2022
Foundation Launching its Largest Natural History Study to Date for 1,500 People with Inherited Retinal Diseases Caused by Rare Mutated Genes
Press ReleasesUni-Rare Study will improve clinical understanding of more IRDs and boost development of potential therapies.
-

Sep 15, 2022
RD Fund Participates in a €75 Million Series B for SparingVision
The Foundation in the NewsProceeds to fund first, in-human trials of breakthrough gene-agnostic therapy products and advance CRISPR-based genome editing portfolio.
-

Jan 29, 2021
Renowned Pioneers in Ophthalmology Join SparingVision’s Scientific Advisory Board
The Foundation in the NewsDevelopment of lead asset SPVN06 to further benefit from high-level Clinical Advisory Board
-

Oct 7, 2020
AGTC Joins the My Retina Tracker® Program as a New Scientific Collaborator
The Foundation in the News -
Sep 24, 2020
Foundation Fighting Blindness To Jointly Host Online Continuing Medical Education (CME, COPE) Webinar
Press ReleasesThe event will review best practices for care and management of patients with inherited retinal diseases such as retinitis pigmentosa, Usher syndrome, and Stargardt disease.
-
Aug 21, 2020
Foundation Fighting Blindness Commits $6.5 Million for New Retinal Disease Research Grants
Press ReleasesNew grants include development of CRISPR/Cas9 gene-editing treatments, new disease models, and a retinal regeneration therapy
-

Aug 4, 2020
Foundation Insights Forum – July 30, 2020
Insights ForumThe Foundation Fighting Blindness is pleased to provide an audio recording and full transcript of the Insights Forum, our quarterly conference call providing updates to the inherited retinal disease community. The call took place on July 30, 2020.
-
Mar 31, 2020
COVID-19 Resources
Foundation NewsThe Foundation Fighting Blindness is closely monitoring the COVID-19 situation and its impact on the IRD community.
-

Feb 14, 2020
Unstoppable: With Her “Trusty Guide Shiloh” By Her Side, Janni Lehrer-Stein Travels the World and Advocates for Disability Rights
The Foundation in the NewsPeople with disabilities are no different from anyone else. We all have strengths and challenges, and we all seek to be fully engaged in society. Treating blind people differently, either as incapable of conducting everyday tasks in life, or conversely, acting as though our ability to conduct even the most mundane chore is remarkable can be discouraging.
-
Feb 6, 2020
Foundation Insights Forum – January 31, 2020
Insights ForumThe Foundation Fighting Blindness is pleased to provide an audio recording and full transcripts of the Insights Forum, our quarterly conference call providing updates to the inherited retinal disease community. The call took place on January 31, 2020.
-

Feb 6, 2020
ProQR Therapeutics Teams Up with the Foundation Fighting Blindness and Blueprint Genetics to Support the My Retina Tracker® Program for People Living with Inherited Retinal Diseases
Press ReleasesMy Retina Tracker Program is the highest volume IRD genetic testing program in the U.S.
-

Nov 27, 2019
Foundation Fighting Blindness Launches Natural History Study for People with RP Caused by EYS Gene Mutations
Press ReleasesKnown as Pro-EYS, the study will help researchers design clinical trials for potential therapies
-
Nov 8, 2019
Foundation Insights Forum – October 30, 2019
Insights ForumThe Foundation Fighting Blindness is pleased to provide an audio recording and full transcript of the Insights Forum, our quarterly conference call providing updates to the inherited retinal disease community. The call took place on October 30, 2019.
-
Oct 2, 2019
Blueprint Genetics, InformedDNA and the Foundation Fighting Blindness launch an open access program for patients with inherited retinal disease in the United States
Press ReleasesThe program will offer patients with inherited retinal disease no-cost genetic testing and genetic counseling in the United States. Look for updated information on how to participate to be posted in mid-October, with program registration starting shortly thereafter.
-
Jun 3, 2019
Tackling the Next Gene Therapy Challenge: Autosomal Dominant Diseases
Foundation NewsA discussion of strategies concerning the development of autosomal dominant disease therapies at the Translational Research Acceleration Program (TRAP) in November 2013.
-

Jul 19, 2018
Foundation Fighting Blindness Urges Congress to Pass ‘Eye-Bonds’ Legislation
Press ReleasesBill Introduced in U.S. House Would Speed Up Cures for Blindness
-

Jun 8, 2018
Foundation Fighting Blindness and CheckedUp® Partner to Educate Retinal-Disease Patients About Research, Resources, and Emerging Therapies During Doctor Visits
Press ReleasesThe Foundation Fighting Blindness (the Foundation) and CheckedUp have formed a collaborative partnership to deliver patient-friendly diagnostic and disease-management information to people with retinal diseases such as age-related macular degeneration, retinitis pigmentosa, and Stargardt disease during their visits to eye doctors.
-

Jan 9, 2018
A Retinal Research Nonprofit Paves the Way for Commercializing Gene Therapies
The Foundation in the NewsAN EMERGING, vision-restoring gene therapy for a devastating retinal disease is poised for Food and Drug Administration (FDA) approval. If it gets the regulatory nod, it will be the first gene therapy to receive FDA approval for the eye or an inherited condition.
Latest Research
-

Apr 12, 2024
Phase 3 Clinical Trial of NAC Launched for RP Patients
Eye On the Cure Research NewsThe drug is thought to work independent of the mutated gene causing RP.
-

Apr 11, 2024
ViGeneron Launches Clinical Trial of Gene Therapy for RP Caused by CNGA1 Mutations
Eye On the Cure Research NewsThe gene therapy is administered using a less-invasive intravitreal injection.
-
.png)
Apr 8, 2024
Ocugen to Launch Phase 3 Clinical Trial of Modifier Gene Therapy for RP
Eye On the Cure Research NewsThe emerging treatment is designed to work independent of the patient’s mutated gene.
-

Mar 27, 2024
Nanoscope Plans to Apply for FDA Approval for its Optogenetic Therapy
Eye On the Cure Research NewsThe company reported positive two-year results for its Phase 2 clinical trial of MCO-010.
-

Feb 21, 2024
jCyte Announces Plans to Launch Phase 3 Clinical Trial of Cell Therapy for RP
Eye On the Cure Research NewsKnown as retinal progenitors, the company’s jCells® are designed to preserve photoreceptors in people with retinitis pigmentosa (RP) and other retinal diseases.
-

Feb 9, 2024
Beacon Therapeutics Reports Encouraging Interim Results from Phase 2 Clinical Trial for XLRP Gene Therapy
Eye On the Cure Research NewsThe company plans to begin a Phase 2/3 clinical trial for the treatment during the first half of 2024.
-
Jan 8, 2024
Retinitis Pigmentosa Research Advances
Retinal Disease Research AdvancesRecent developments in research on retinitis pigmintosa.
-

Nov 8, 2023
Kiora Reports Vision Restoration in Phase 1/2 Clinical Trial for Photoswitch Therapy
Eye On the Cure Research NewsThe company’s molecule is designed to restore some vision to people with ultra-low or no vision caused by advanced retinitis pigmentosa and other retinal diseases.
-

Nov 2, 2023
New Report: Vitamin A Supplementation Provides No Vision Benefit to RP Patients
Eye On the Cure Research NewsThe report also concludes that vitamin E supplementation accelerates vision loss for RP patients
-
.png)
Aug 31, 2023
SparingVision Recruiting for Clinical Trial of Cone-Preserving Gene Therapy for RP
Eye On the Cure Research NewsFirst cohort of patients has been dosed in Phase ½ clinical trial taking place in Pittsburgh and Paris
-

Jul 12, 2023
PYC Doses First Patient in Clinical Trial of RNA Therapy for RP11 (PRPF31 Mutations)
Eye On the Cure Research NewsThe emerging RNA therapy is designed to boost expression of the PRPF31 protein
-

Jun 12, 2023
Beacon Therapeutics to Advance XLRP, Cone-Rod Dystrophy, and Dry AMD Gene Therapies
Eye On the Cure Research NewsAn emerging XLRP gene therapy acquired from AGTC is the company’s lead clinical program
-

Jun 1, 2023
Coave Reports Encouraging Phase 1/2 Clinical Trial Results for PDE6B Gene Therapy
Eye On the Cure Research NewsThe company is expanding the trial to enroll younger patients with less advanced disease
-

Apr 28, 2023
Budd Tucker Honored with ARVO’s Cogan Award for Advancements in Retinal Therapy Development and Manufacturing
Eye On the Cure Research NewsDr. Tucker is leading the development of GMP facilities for manufacturing clinical grade retinal therapies
-

Apr 21, 2023
Bionic Sight Reports Meaningful Vision Improvements for RP Patients Receiving Highest Dose of its Emerging Optogenetic Therapy
Eye On the Cure Research NewsClinical trial participants with advanced vision loss from RP were able to identify fruits and vegetables
-

Mar 30, 2023
Vision Improvements Reported for RP Patients in Phase 2B Clinical Trial of Nanoscope’s Optogenetic Therapy
Eye On the Cure Research NewsMost participants with advanced retinitis pigmentosa (RP) in the trial had improved navigation and/or object discrimination in reduced lighting conditions
-

Jan 3, 2023
Opus Genetics Acquires Rights to Gene Therapies for BEST1 and RP (RHO)
Eye On the Cure Research NewsThe company plans to seek clinical trial authorization for the BEST1 gene therapy during the second half of 2023
-
.png)
Dec 2, 2022
SparingVision Receives Authorization to Launch US Clinical Trial for its Cone-Preserving Treatment
Eye On the Cure Research NewsThe emerging therapy is designed to work independent of the mutated gene causing retinitis pigmentosa
-

Jul 25, 2022
Nanoscope Doses First Patient in Phase 2 Clinical Trial of its Optogenetic Therapy for Stargardt Disease
Eye On the Cure Research NewsThe emerging therapy is designed to restore vision for people with advanced retinal degenerative diseases
-
.png)
Jul 14, 2022
Endogena Launches Clinical Trial of Therapy to Activate Stem Cells in RP Patients’ Retinas
Eye On the Cure Research NewsThe emerging treatment is gene-agnostic.
-
Apr 25, 2022
Webinar for the X-Linked Retinitis Pigmentosa (XLRP) Community
Science EducationThe Foundation Fighting Blindness recently held a webinar to provide details about an upcoming Externally-Led Patient Focused Drug Development (EL-PFDD) meeting for XLRP, and to provide background information about the Food and Drug Administration’s (FDA) drug review process.
-

Mar 15, 2022
Aldeyra Launches Phase 2 Clinical Trial of Methotrexate Intravitreal Injections for RP
Eye On the Cure Research NewsLab studies showed the drug can effectively address the misfolded rhodopsin protein
-
.png)
Jan 19, 2022
ReNeuron Not Continuing Clinical Development of Cell-Based Therapy for RP
Eye On the Cure Research NewsCompany seeks to out-license its retinal progenitor therapy to a partner
-

Dec 17, 2021
ProQR Doses First Patients in Phase 2/3 Clinical Trials for its USH2A-Exon 13 RNA Therapy
Eye On the Cure Research NewsThe Sirius trial is for USH2A (exon 13 mutations) patients with advanced vision loss. The Celeste trial is for USH2A (exon 13 mutations) patients with moderate to early vision loss.
-
.png)
Dec 13, 2021
Ocugen to Launch Clinical Trial for Cross-Cutting RP Gene Therapy
Eye On the Cure Research NewsThe clinical trial is for RHO and NR2E3 mutations, but the retinal gene therapy has the potential to benefit people with a variety of other mutated genes
-

Sep 9, 2021
Optogenetics: Hope for Vision Restoration for Advanced Retinal Diseases
Eye On the Cure Research NewsEarly, encouraging results from two human studies — trials launched by Bionic Sight and GenSight — are putting optogenetic therapies in the spotlight for patients with advanced vision loss from retinal conditions.
-

May 24, 2021
Investigators Report Partial Vision Restoration for One Patient in Optogenetic Therapy Trial
Eye On the Cure Research NewsThe gene-agnostic approach is designed to restore some vision to people with advanced vision loss
-

May 14, 2021
Biogen’s Phase 2/3 Clinical Trial for XLRP Gene Therapy Doesn’t Meet Primary Endpoint
Eye On the Cure Research NewsMore details from the clinical trial will be reported at a later date
-

May 10, 2021
AGTC Continues to Report Encouraging Data from its Phase 1/2 XLRP Gene Therapy Clinical Trial
Eye On the Cure Research NewsThe company is planning the launch of a Phase 2/3 trial for its XLRP gene therapy
-
May 5, 2021
ARVO 2021 Highlight: CRISPR/Cas9 Therapy Emerging for Dominant RP Caused by RP1 Mutations
Eye On the Cure Research NewsGene-editing approaches are often better suited for autosomal dominant retinal diseases than gene replacement therapies
-

May 3, 2021
ARVO 2021 Highlight: Update on Clinical Trial of jCyte’s Cellular Therapy for RP
Eye On the Cure Research NewsCellular treatment provided significant improvements in visual acuity for subpopulation of patients with better vision
-
.png)
Apr 26, 2021
SparingVision to Acquire Therapy for Resurrecting Dormant Cones for Vision Restoration
Eye On the Cure Research NewsThe gene-agnostic approach shows promise for people with late-stage RP and related diseases.
-
Apr 9, 2021
Foundation Invests $5.5 Million in Seven New Translational Research Projects
Eye On the Cure Research NewsProjects target a variety of conditions including: age-related macular degeneration, Stargardt disease, retinitis pigmentosa, and Usher syndrome type 3A
-

Mar 30, 2021
Bionic Sight’s Optogenetic Therapy Enables Blind Patients to Detect Light and Motion in Early Trial
Eye On the Cure Research NewsThe approach holds potential for restoring vision to people with little or no vision
-

Nov 18, 2020
AGTC Announces Results for Achromatopsia Gene Therapy Clinical Trials
Eye On the Cure Research NewsThe company will continue enrolling younger patients in higher dosing groups.
-

Oct 21, 2020
SparingVision Raises €44.5 Million for Gene-Independent RP Therapy
Eye On the Cure Research NewsThe company is planning a clinical trial for its emerging neuroprotective treatment in 2021
-
Oct 20, 2020
RP Treatment Derived from Induced Pluripotent Stem Cells Advances into Clinical Trial
Eye On the Cure Research NewsThe stem cells were derived from mature blood cells and coaxed to become photoreceptors
-

Aug 4, 2020
4D Molecular Therapeutics Launches Phase 1 Clinical Trial for Choroideremia Gene Therapy
Eye On the Cure Research NewsGene therapy delivered by intravitreal injection
-

Jul 30, 2020
Researchers Identify Regions in the Retina to Target Therapies for Certain RP Patients
Eye On the Cure Research NewsUniversity of Pennsylvania investigators studied retinas of patients and canines with retinitis pigmentosa caused by mild mutations in RHO
-

Jul 27, 2020
jCyte Reports Promising Results for Phase 2b Clinical Trial of its Cellular Therapy for RP
Eye On the Cure Research NewsThe emerging therapy is designed to work independent of the mutated gene causing vision loss
-

Jul 22, 2020
AGTC Planning Phase 2/3 Clinical Trial for XLRP Gene Therapy
Eye On the Cure Research NewsThe company is also expanding its Phase ½ trial for the emerging treatment
-

Jul 7, 2020
Biogen Entering into Licensing Agreement with Mass Eye and Ear to Develop PRPF31 Gene Therapy
Eye On the Cure Research NewsThe goal of the partnership is to advance a PRPF31 gene therapy into a clinical trial
-

Jun 18, 2020
Bionic Sight Doses First Patient in Clinical Trial for Optogenetic Therapy
Eye On the Cure Research NewsTreatment combines gene therapy and a device that generates and delivers retinal code
-

May 21, 2020
Nacuity’s Emerging Anti-Oxidative Therapy Moves into Clinical Trial
Eye On the Cure Research NewsThe oral treatment shows promise for slowing vision loss in people with RP and Usher syndrome, regardless of genetic profile
-

May 13, 2020
jCyte Enters into Licensing Agreement with Santen Pharmaceutical for Cell Therapy
Eye On the Cure Research NewsEmerging treatment designed to preserve vision for people with RP and related conditions
-

Feb 27, 2020
Researchers Report Six-Month Results from Biogen-Sponsored XLRP Gene Therapy Clinical Trial
Eye On the Cure Research NewsVision improvements observed with medium and high doses
-

Feb 7, 2020
Genetic Testing for Inherited Retinal Diseases through the Foundation’s Open Access Program
Science EducationThe benefits of genetic testing for IRD patients, how to participate in the Foundation’s Open Access program, and what to expect from the genetic testing process.
-

Jan 10, 2020
AGTC Reports Positive Six-Month Results for XLRP Phase 1/2 Gene Therapy Trial
Eye On the Cure Research NewsThe company is planning a Phase 3, pivotal trial for end of 2020
-

Dec 16, 2019
First Patient Receives ProQR’s AON Therapy in Clinical Trial for RP Caused by RHO-P23H Mutation
Eye On the Cure Research NewsThe trial becomes ProQR’s third human study for inherited retinal disease therapies
-

Nov 15, 2019
FDA Authorizes Stem Cell Clinical Trial for RP in Los Angeles
Eye On the Cure Research NewsPhase 1/2a human study will evaluate neural progenitors for preserving vision
-

Nov 7, 2019
AGTC Announces Development of Stargardt Disease Gene Therapy
Eye On the Cure Research NewsDual-vector delivery system designed to deliver the large ABCA4 gene
-

Oct 18, 2019
ReNeuron Reports Interim Results for Eight Patients with RP in Phase 2a Trial for Stem Cell Therapy
Eye On the Cure Research NewsThe Foundation funded earlier lab studies that made the clinical trial possible.
-

Oct 1, 2019
AGTC Reports Promising Interim Results for XLRP and Achromatopsia Gene Therapy Trials
Eye On the Cure Research NewsAGTC used Foundation’s My Retina Tracker registry to recruit patients for trials
-

Aug 14, 2019
ProQR Receives Authorization to Begin Clinical Trial for Autosomal Dominant Retinitis Pigmentosa Treatment
Eye On the Cure Research NewsThe treatment focuses on the P23H mutation in the RHO gene, which is the most prevalent variant causing adRP in the US and affects approximately 2,500 people.
-

Jul 9, 2019
SparingVision Gets EU Funding Boost for Development of Cross-Cutting Gene Therapy
Eye On the Cure Research NewsA clinical trial for the treatment, designed to preserve cone photoreceptors, is planned in the US and Europe in 2020.
-
Jun 17, 2019
The Retina is a Proving Ground for a Broad Range of Neurological Therapies
Science EducationRetinal research paves the way for new treatments for the entire neurological system.
-

May 20, 2019
Dr. Don Zack Honored for Research Contributions by ARVO and the Foundation Fighting Blindness
Eye On the Cure Research NewsDr. Zack is a member of the Foundation’s Scientific Advisory Board and chairs its Cellular Molecular Mechanisms of Disease study section.
-

May 9, 2019
Eye Bonds Re-Introduced to New Congress: Potentially $1 Billion in Government-Backed Funding for Eye Research
Eye On the Cure Research NewsEye Bonds provide the opportunity to advance, and accelerate development for, more promising treatments into and through clinical trials and out to the people who need them.
-
May 1, 2019
ARVO 2019: Emerging Drug for RP Evaluated in Safety & Tolerability Study
Eye On the Cure Research NewsFrancois Paquet-Durand, PhD, chief scientific officer at the company Mireca, discusses an emerging drug for retinitis pigmentosa, and other inherited retinal diseases.
-

Mar 12, 2019
First Patient Receives ProQR’s Emerging USH2A Therapy in Clinical Trial
Eye On the Cure Research NewsProQR, a developer of RNA therapies in the Netherlands, announced that the first clinical-trial participant has received its emerging treatment, which targets retinitis pigmentosa and Usher syndrome caused by mutations in exon 13 of the USH2A gene.
-

Feb 21, 2019
Encouraging Vision Improvements Reported in ReNeuron's Cell-Therapy Clinical Trial
Eye On the Cure Research NewsReNeuron, a cellular therapy developer in the UK, has reported vision improvements in the treated eyes of the first three retinitis pigmentosa (RP) patients in the Phase II part of the Phase I/II clinical trial for its proprietary human retinal progenitor cells (hRPC). The Phase I portion of the trial, completed last year, primarily assessed safety in subjects with minimal remaining vision.
-

Jan 29, 2019
The Foundation Receives a $100,000 Research Grant from Sofia Sees Hope
Eye On the Cure Research NewsSofia Sees Hope, a nonprofit dedicated to finding treatments and cures for people with Leber congenital amaurosis (LCA) and other inherited retinal diseases (IRDs), has made a $100,000 donation to the Foundation Fighting Blindness to support therapy development and genetic testing.
-
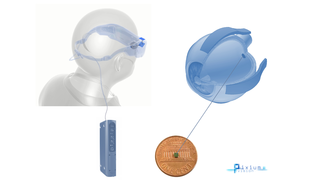
Jan 17, 2019
Pixium's PRIMA Bionic Vision System Restores Central Vision in Dry AMD Clinical Trial
Eye On the Cure Research NewsThe French bioelectronics company Pixium Vision has reported that its PRIMA bionic vision system has restored some central vision in patients with advanced dry age-related macular degeneration (AMD) participating in a clinical feasibility trial.
-

Dec 4, 2018
ProQR Receives FDA Authorization to Launch Clinical Trial for USH2A Therapy
Eye On the Cure Research NewsProQR, a biotech in the Netherlands developing therapies for rare diseases, has received authorization from the US Food and Drug Administration to launch a Phase I/II clinical trial for QR-421a, its treatment targeting mutations in exon 13 of the USH2A gene.
-

Nov 2, 2018
Foundation Invests $2.5 Million in Search for Elusive Retinal Disease Genes and Mutations
Eye On the Cure Research NewsSince 1989 genetic researchers, many funded by the Foundation, have identified approximately 270 genes linked to IRDs. In most cases, defects in a single gene can cause a retinal disease and vision loss.
-

Sep 11, 2018
FFB Congratulates RPE65 Gene Therapy Researchers for Champalimaud Award
Eye On the Cure Research NewsOn September 4, 2018, seven researchers, including six previously funded by the Foundation, were recognized with the prestigious 2018 Antonio Champalimaud Vision Award for their contributions to the advancement of blindness-reversing RPE65 gene therapies.
-

Aug 22, 2018
Ophthotech is Advancing an Impressive Portfolio of Cutting-Edge Therapies for Retinal Diseases
Eye On the Cure Research NewsThe company is taking on a multi-track strategy that includes retinal gene-therapy development, including delivery of over-sized genes and design of a two-step process of gene knockdown and replacement for autosomal dominant conditions.
-
Aug 15, 2018
FFB Provides Four Career Development Awards to Up-and-Coming Clinical Researchers
Eye On the Cure Research NewsEach recipient will receive a total of $375,000 over five years to help build an independent research program in addition to their clinical practices.
-
Aug 6, 2018
FFB Funding More than $2 Million in New Research
Eye On the Cure Research NewsSeventy scientists submitted requests for funding.
-

Jul 20, 2018
Call to Action: Ask Congress to Support $1 Billion in Eye Research
Eye On the Cure Research NewsCall to Action: Ask Congress to Support $1 Billion in Eye Research
-
Jul 5, 2018
Retinal Regeneration: Releasing Your Inner Salamander
Eye On the Cure Research NewsMany research groups from around the world are investigating ways to create new photoreceptors from stem cells for transplantation into the retina for vision restoration.
-
Jun 22, 2018
VISIONS2018 Live Stream
Eye On the Cure Research NewsWatch recorded sessions from VISIONS2018.
-

May 30, 2018
French Gene Therapy Company Advancing Three Programs for Retinal Diseases
Eye On the Cure Research NewsHorama’s gene therapies are injections of healthy copies of a gene underneath the retina to compensate for the defective gene.
-
May 7, 2018
ARVO 2018: Dr. Henry Klassen Provides Update on jCyte Stem Cell Trials
Eye On the Cure Research NewsDr. Henry Klassen, jCyte co-founder and investigator at UC Irvine, provides an update on the clinical trials for an RP therapy derived from stem cells.
-

May 3, 2018
ARVO 2018: Dr. Stephen Daiger Reports on the State of Genetic Testing for Inherited Retinal Diseases
Eye On the Cure Research NewsARVO 2018: Dr. Stephen Daiger Reports on the State of Genetic Testing for Inherited Retinal Diseases
-

May 2, 2018
ARVO 2018: Dr. Steve Rose Reports on CRISPR/Cas9 for Inherited Retinal Diseases
Eye On the Cure Research NewsFFB’s own Dr. Steve Rose, chief scientific officer, reviews our commitment to funding and exploring CRISPR/Cas9 gene editing for inherited retinal disease.
-

Apr 25, 2018
ARVO 2018: World's Largest Show and Tell for Innovations in Eye Research
Eye On the Cure Research NewsMore than 11,000 eye researchers from around the world — including five intrepid members from FFB’s science team — will gather to participate in what is essentially a massive “show and tell” of the latest scientific advancements.
-

Apr 9, 2018
Study Suggests Vitamin A May Benefit Children with RP
Eye On the Cure Research NewsAn FFB-funded study at Massachusetts Eye andEar Infirmary (MEEI) suggests that vitamin A palmitate supplementation may slow the decline of cone function by nearly 50 percent in children with retinitis pigmentosa (RP).
-

Feb 14, 2018
FFB-CRI Investing $7.5 Million in Emerging Therapy for USH2A
Eye On the Cure Research NewsThe Foundation Fighting Blindness Clinical Research Institute (FFB-CRI) has entered into a partnership with ProQR to develop a retinal therapy for people with Usher syndrome type 2A (USH2A) caused by mutations in exon 13 of the USH2A gene.
-

Feb 7, 2018
AGTC Launches XLRP Gene Therapy Clinical Trial at Five Sites in U.S.
Eye On the Cure Research NewsTaking place at five locations in the United States, the clinical trial will enroll approximately 15 males with X-linked retinitis pigmentosa caused by mutations in the gene RPGR and will primarily evaluate safety.
-
Jan 17, 2018
Clinical Trial to Launch for System Combining Optogenetics and Eyewear
Eye On the Cure Research NewsThe French biotech GenSight Biologics has received regulatory authorization in the UK to launch the PIONEER Phase 1 \ 2 clinical trial for its GS030 system — a light-sensing gene therapy (optogenetics) coupled with eyewear, which enhances visual stimulation.
-

Jan 9, 2018
Top Retinal Research Advances for 2017
Eye On the Cure Research NewsAn exciting year in fighting blindness.
-
Dec 21, 2017
jCyte Reports Results for Phase 1/2a Clinical Trial for Retinal-Cell Treatment
Eye On the Cure Research NewsSome participants reported increased light sensitivity, improved color vision, better mobility, and improved reading ability.
-
Dec 20, 2017
History Is Made: FDA Approves Spark's Vision-Restoring Gene Therapy
Eye On the Cure Research NewsKnown as LUXTURNA™ (voretigene neparvovec), the gene therapy restored vision in a clinical trial for people between the ages of 4 and 44 with Leber congenital amaurosis (LCA) caused by mutations in the gene RPE65.
-
Nov 21, 2017
Stem-Cell Therapy Clinics Remain Inadequately Regulated, Pose Risk to Patients
Eye On the Cure Research NewsIf a clinic is charging for a stem-cell treatment or procedure for an IRD, it is probably not legit. The expense to the patient is a major red flag.
-

Oct 13, 2017
FDA Committee Unanimously Recommends Approval for Spark's RPE65 Gene Therapy - Final Decision Due in January 2018
Eye On the Cure Research NewsAn advisory committee comprised of FDA-selected experts voted unanimously – 16 to 0 – to recommend approval.
-

Sep 27, 2017
The Foundation's Investments Are Filling the Pipeline for Vision-Saving Therapies
Eye On the Cure Research NewsIn addition to funding promising biotech start-ups, the Foundation Fighting Blindness has played a critical role in developing research talent.
-
Aug 30, 2017
MeiraGTx Treats First Patient in XLRP Gene-Therapy Trial
Eye On the Cure Research NewsThe MeiraGTx gene therapy involves injection of healthy copies of RPGR underneath the retina. The RPGR copies are contained in a human-engineered virus — known as an adeno-associated virus or AAV — designed to readily penetrate retinal cells to deliver the therapeutic genetic cargo.
-
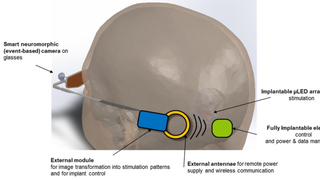
Jul 26, 2017
Scientists Receive $25 Million to Develop a Vision-Restoring System that Connects to the Brain
Eye On the Cure Research NewsThe high-tech, vision-restoring system interfaces with the visual cortex, the back of the brain where visual input is processed to create the images we see.
-
Jul 25, 2017
Foundation Fighting Blindness and 4D Molecular Therapeutics Partner to Boost Retinal Gene Therapy Development
Eye On the Cure Research NewsThe partnership will help companies and researchers quickly obtain and implement high-quality vectors for their retinal gene-therapy development efforts.
-
Jul 24, 2017
FFB-Funded Scientists Report on Nine Promising Translational Research Efforts
Eye On the Cure Research NewsThe Foundation Fighting Blindness has taken the translational challenge head on by investing more than $75 million in therapy-development projects with strong clinical-trial potential through its Translational Research Acceleration Program (TRAP), which includes Gund-Harrington Scholar Awards.
-

Jul 12, 2017
SparingVision Formed to Advance Sight-Saving Protein for RP
Eye On the Cure Research NewsSparingVision received a €300,000 award known as the Honor Prize from the French Ministry of Research. The award is given to new, innovative companies in France competing in a national contest.
-
Jun 29, 2017
Researchers Find Mutation as Frequent Cause of RP in American Hispanics
Eye On the Cure Research NewsThe discovery can help genetic experts diagnose more patients with adRP, and it gives researchers a target for developing potential therapies.
-
Jun 1, 2017
Valproic Acid's Effect Too Small in One-Year Clinical Trial
Eye On the Cure Research NewsWhile a therapy for adRP will not emerge from the clinical trial, study investigators advanced development of a new outcome measure known as EZ Area to quickly and accurately evaluate potential therapies for RP in human studies.
-

May 23, 2017
Forty-Four High-Impact Retinal-Research Efforts Highlighted at FFB-Casey Innovation Summit
Eye On the Cure Research NewsIn its fourth year, the meeting is becoming the world’s most comprehensive overview of the promising research underway for emerging IRD treatments.
-

May 8, 2017
FFB Funding Helps Retinal Genetics Lab Secure $2 Million Investment
Eye On the Cure Research NewsHow the Foundation Fighting Blindness (FFB) provided timely funding of $155,000 to help a lab at the University of California, San Diego (UCSD), leverage a $2 million retinal-gene discovery project.
-
Apr 28, 2017
jCyte Stem-Cell Therapy Moves into Phase IIb Clinical Trial for RP
Eye On the Cure Research NewsBased on lab studies, researchers believe the treatment can preserve and potentially rescue the patient’s existing photoreceptors, thereby saving and possibly restoring vision.
-

Apr 4, 2017
FFB-CRI Launching Natural History Study for People with USH2A Mutations
Eye On the Cure Research NewsThe study — known as RUSH2A (“R” stands for “rate of progression”) — is beginning in spring 2017 and will take place at about 20 clinical sites around the world. RUSH2A investigators will use a variety of technologies to monitor changes in vision and retinal structure to document and analyze disease progression.
-

Mar 22, 2017
Dr. Eliot Berson, Pioneer in Vitamin A Therapy for Retinitis Pigmentosa, Passes Away
Eye On the Cure Research NewsDr. Berson dedicated himself to clinical care and vision-saving research for people with inherited retinal diseases for five decades.
-
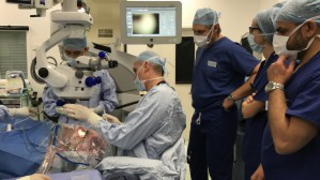
Mar 20, 2017
First Patient Treated in XLRP Gene Therapy Clinical Trial
Eye On the Cure Research NewsThe Nightstar gene therapy involves injection of healthy copies of RPGR underneath the retina.
-
Mar 16, 2017
Unregulated Stem-Cell Therapy Causes Severe Vision Loss for Three Florida Women
Eye On the Cure Research News“…participation in a study for an emerging therapy that is not regulated by the FDA or another well-recognized regulatory agency like the European Medicines Agency in Europe, is fraught with dangers and can lead to unexpected serious consequences.”
-
Feb 17, 2017
AGTC Leverages Funding from the Foundation to Move Promising Treatments into Clinical Trials
Eye On the Cure Research NewsCompany Builds on FFB’s Initial Investment to Garner $265 Million in Therapy Development Funding
-

Jan 19, 2017
Foundation Investing in Drug to Slow Many Forms of RP
Eye On the Cure Research NewsThe Foundation Fighting Blindness Clinical Research Institute (FFB-CRI) has announced an investment of up to $7.5 million to advance the potential therapy into and through a Phase II clinical trial.
-
Dec 21, 2016
FFB-CRI Leads Effort to Identify Outcome Measures for Therapies in Clinical Trials
Eye On the Cure Research NewsImproved outcome measures will make clinical trials for degenerative retinal diseases — including age-related macular degeneration (AMD), the world’s leading cause of blindness in seniors, and inherited retinal conditions such as RP and Stargardt disease — less expensive to conduct and able to deliver more precise results.
-
Dec 19, 2016
A Change in Identity Might Someday Save Vision
Eye On the Cure Research NewsBy changing the identity of cells in the retina–namely rods–researchers may someday be able to slow or halt vision loss for those with retinitis pigmentosa (RP) and other related conditions.
-
Oct 18, 2016
Building a Wiring Diagram for the Retina to Help Researchers Save and Restore Vision
Eye On the Cure Research NewsUnderstanding the pathways of the retinal neural network — and how they are rewired with aging and disease — is helpful in trying to save and restore vision.
-

Oct 11, 2016
Nobel-Prize-Winning Stem-Cell Researcher Delivers Keynote at FFB-Funded Conference in Kyoto
Eye On the Cure Research NewsDr. Shinya Yamanka discussed his early clinical trial for iPSC-derived retinal pigment epithelial (RPE) cells for a 78-year-old woman with advanced wet age-related macular degeneration (AMD).
-
.png)
Oct 6, 2016
Embrace Your Exceptions: A Mantra for Understanding Retinal-Disease Inheritance
Eye On the Cure Research NewsThe complex and elusive nature of these conditions can also extend to the way they are passed down in families, making diagnosis and prognosis quite challenging.
-

Aug 18, 2016
Optogenetic Therapy Takes First Step Forward in Clinical Trial
Eye On the Cure Research NewsRetroSense’s optogenetic therapy is designed to restore vision to people who are completely blind from retinal degenerative diseases such as retinitis pigmentosa by bestowing light sensitivity to retinal ganglion cells, which survive after photoreceptors, the cells that make vision possible, are lost.
-

Aug 2, 2016
Pixium Vision Reports Progress in Development of Two Advanced Bionic Retina Systems
Eye On the Cure Research NewsBoth approaches show strong, near-term potential for providing meaningful vision to people who are otherwise blind from retinal diseases such as retinitis pigmentosa and age-related macular degeneration (AMD).
-

Jul 21, 2016
Stem-Cell Therapy for Retinitis Pigmentosa Safe Thus Far in Early Human Study
Eye On the Cure Research NewsThe trial is one of the first-ever for a stem-cell-derived therapy for RP.
-
Jul 1, 2016
VISIONS 2016 — Dr. Shomi Bhattacharya Wins FFB Award for Gaining an Understanding of Variations in Vision Loss
Eye On the Cure Research NewsAt VISIONS 2016, FFB’s national conference, the Foundation honored him with its Ed Gollob Board of Directors Award for breakthrough research conducted within the past year.
-

Jul 1, 2016
VISIONS 2016 - Dr. Richard Weleber Receives FFB's Highest Research Honor, Recognized in Touching Video
Eye On the Cure Research NewsDr. Weleber became the 10th recipient of the Foundation’s highest honor, named after FFB co-founder Lulie Gund, during the opening lunch of the VISIONS 2016 conference.
-
Jun 24, 2016
A Steady Hand in Saving Vision
Eye On the Cure Research NewsSubretinal injection is the most common form of delivery for gene therapies currently in clinical trials.
-

Oct 8, 2015
A Leap Forward: Spark Therapeutics Seeks FDA Approval for its Vision-Restoring Gene Therapy
Eye On the Cure Research News -
Jun 27, 2015
VISIONS 2015 — Dr. José Sahel Receives Foundation's Most Prestigious Research Honor
Eye On the Cure Research NewsFor those of us supporting the drive for vision-saving treatments and cures, he’s exactly the type of person we want on our team.
-

Jun 26, 2015
VISIONS 2015 — Dr. Shannon Boye Receives FFB Award for Excellence in Gene-Therapy Research
Eye On the Cure Research NewsDr. Boye received the Foundation’s Board of Director’s Award, which was presented at VISIONS 2015, FFB’s annual conference, for achievements in retinal research.
-
May 19, 2015
ARVO 2015 Highlight: The National Eye Institute Invests $4 Million in Audacious-Goals Research
Eye On the Cure Research NewsThe mission of the program—to regenerate the neurons and neural connections in the eye and visual system—is synonymous with the Foundation’s mission to eradicate retinal diseases.
-

May 13, 2015
ARVO 2015 Highlight: New Research Boosts Prospects for Saving Vision with RdCVF
Eye On the Cure Research NewsAfter years of refinement and testing in animal models, the emerging therapy is about a year and a half from moving into a clinical trial.
-
May 12, 2015
ARVO 2015 Highlight: A Cut-and-Paste Approach to Fixing Retinal-Disease Genes
Eye On the Cure Research NewsOne of the hot topics at ARVO this year is a rapidly advancing gene-therapy approach called clustered regularly interspaced short palindromic repeats, or CRISPR.
-

Aug 1, 2014
How Evolution is Leading to Gene Therapies for More Retinal Diseases
Eye On the Cure Research NewsAn innovative genetic-engineering approach called “directed evolution” to find optimal gene-delivery systems based on adeno-associated viruses (AAVs).
-

Jul 18, 2014
Despite Blindness, the Peaches are Sweet in Paran
Eye On the Cure Research NewsThe story of vision loss in a Peruvian village
-
Jun 21, 2014
VISIONS 2014 — My Retina Tracker: Track Your Vision and Drive the Research
Eye On the Cure Research NewsThe powerful and secure system enables patients to keep track of their clinical care and vision changes. At the same time, it enables scientists to search the “de-identified” (i.e., anonymous) patient information to study conditions and identify targets for treatments, preventions and cures.
-
Jun 21, 2014
VISIONS 2014 — The Multi-Talented Dr. Shannon Boye
Eye On the Cure Research NewsDr. Boye and her research team received a $900,000 grant for a gene therapy project targeting Leber congenital amaurosis.
-
May 8, 2014
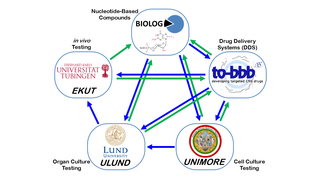
ARVO 2014: European Collaboration Developing Cross-Cutting, Vision-Saving Therapies
Eye On the Cure Research NewsSimply put, they’re creating therapies that can save vision in as many people as possible, independent of the genetic cause of disease.
-
Apr 8, 2014
Total Blindness and Non-24 Sleep Disorder
Eye On the Cure Research NewsNon-24 is a very rare condition affecting many (but not all) people who are totally blind and have absolutely no light perception. Their circadian clocks become out of sync as a result.
-

Mar 20, 2014
UCI Stem-Cell Pioneer Poised to Launch Clinical Trial for RP Patients
Eye On the Cure Research NewsDr. Henry Klassen’s progenitor-based therapy has the potential to rescue a variety of retinal cells — including rods, cones, retinal pigment epithelium and ganglion cells — and, therefore, may save vision in people with a wide range of conditions.
-
Dec 31, 2013
Nouvelle Lumière: French Bionic Retina in a Human Study
Eye On the Cure Research NewsThe French retinal implant developer Pixium quietly launched a clinical trial for its Intelligent Retinal Implant System 1 (IRIS1) in France, Austria and Germany.
-
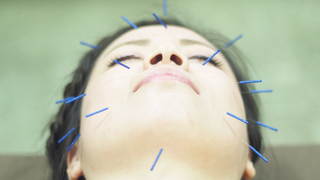
Nov 5, 2013
Is Acupuncture a Beneficial Treatment for Retinitis Pigmentosa?
Eye On the Cure Research NewsAcupuncture definitely has potential benefits, and the breadth of those is being aggressively explored.
-

Oct 30, 2013
Urine Test Helps Diagnose RP Caused by DHDDS Mutations
Eye On the Cure Research NewsWhile it isn’t a substitute for a diagnosis through genetic testing, the urine test can verify that a given DHDDS mutation is, in fact, causing RP.
-
Sep 26, 2013
New Imaging Technique May Be Game-Changer for RP Clinical Trials
Eye On the Cure Research NewsKnown as EZ Width, it holds potential for reducing the time, cost and number of patients needed to determine if a therapy is working in a clinical trial.
-
Aug 9, 2013
When a Condition is More than a Retinal Disease
Science EducationThe Foundation Fighting Blindness is, of course, all about finding treatments and cures for retinal degenerative diseases. However, we are well aware that many of our constituents and their families are dealing with more than just vision loss. That’s because genetic defects causing retinal conditions can sometimes affect other parts of the body. The result is conditions often referred to as syndromes.
-
Jul 26, 2013
Researchers Move Closer to Getting a Complete Genetic Picture of the Retina
Eye On the Cure Research NewsIdentifying the genes and proteins that play a major role in retinal health and vision is an important step in finding preventions and cures for degenerative diseases.
-
Jun 12, 2013
Patient Registries Help Advance Research for Rare Diseases
Eye On the Cure Research NewsMany registries enable patients to collect and track information about their health, so they can take an active role in managing their care.
-
May 10, 2013
Grow Your Own: Harnessing Muller Glia for Retinal Regeneration
Eye On the Cure Research NewsThere’s hope for retinal regeneration for humans, thanks to Foundation-funded researcher Dr. Thomas Reh, who is investigating how to derive new photoreceptors from retinal cells called Muller glia.
-
May 7, 2013
Retinal Regeneration is Major Focus of NEI's Audacious Goal
Eye On the Cure Research NewsThe goal, “to regenerate the neurons and neural connections in the eye and visual system,” is exactly what people with retinal diseases need to save and restore their vision.
-

Apr 30, 2013
Researcher Revolutionized Fight Against Blindness and Cancer
Eye On the Cure Research NewsA profile on Dr. Robert Langer, a medical researcher who has received dozens of awards, accolades and honorary degrees, including, recently, FFB’s Visionary Award.
-
Mar 8, 2013
Staying Alive: Saving Retinal Cells to Preserve Vision
Science EducationSometimes, saving vision simply comes down to keeping retinal cells alive, or at least slowing their degeneration.
-

Feb 18, 2013
History in the Making
Eye On the Cure Research NewsMore good news about treatments and technological advances for restoring vision for people with retinal diseases.
-
Jun 19, 2012
Have I Got a Cure for You! Debunking an Alleged Treatment on the Internet
Eye On the Cure Research NewsHow do you know if a treatment is legit? There should be preclinical and clinical trial data published in a peer-reviewed journal on research for the treatment.
-
Oct 4, 2011
Gene Therapy Revives Cones Long After They Stop Working
Eye On the Cure Research NewsA Foundation-funded research group is developing a gene therapy that revives degenerating cones, enabling them to regain their ability to respond to light and provide vision.
Related Resources
-

Apr 8, 2024
What Does “Blindness” Really Mean?
Beacon StoriesJenny Schisler has retinitis pigmentosa. Jenny wants to spread awareness of what having a visual impairment really means to her and others affected by retinal degenerative diseases.
-

Oct 16, 2023
Finding the “Keys to the Kingdom”
Beacon StoriesTricia Waechter, who has retinitis pigmentosa (RP), refers to her white canes as the “keys to the kingdom.” Using a white cane has opened a whole new world for Tricia, and that is why her company, Blind Girl Designs, includes white canes on many of their apparel designs.
-
Aug 21, 2023
Life as a Teen with Retinitis Pigmentosa
Beacon StoriesAt a mere 15 years old, Ava Ruggiero’s world shifted when she received a diagnosis of retinitis pigmentosa. As Ava continues to find balance as a student and athlete, she discusses the challenges of navigating her diagnosis alongside her parents, Joseph and Stephanie.
-

May 12, 2023
Wine for a Cure
DIY Campaign Success StoriesLindsey Blankenship, president of the Foundation Fighting Blindness Colorado Chapter, has created a one-of-a-kind Raising Our Sights Event. Wine for a Cure aims to spread awareness and fundraise on behalf of the Foundation Fighting Blindness with two signature wines from the Oregon Winery, Stoller Family Estates. The featured wines are a Chardonnay named “Variant” and a Pinot Noir named “Pigmentosa.”
-
.png)
Apr 26, 2023
Traveling With a Guide Dog: Advice from a Seasoned Traveler
Beacon StoriesInternational Guide Dog Day, April 26th, is extra special to Janni Lehrer-Stein, as she’s celebrating the 5th anniversary of graduating with “the world’s best guide dog,” Shiloh. Janni, who has retinitis pigmentosa, has traveled to five countries outside of the United States with Shiloh and is sharing advice for traveling with a guide dog.
-

Feb 14, 2023
A Love Without Limits: How John and Min Conquer Blindness Together
Beacon StoriesIn honor of Valentine’s Day, the Foundation Fighting Blindness brings you a special edition of our Beacon Story series, which covers the unique journey of visually impaired couple John and Min.
-

Jan 9, 2023
Fearless Sister Duo Finding Hope
Beacon StoriesApril LuFriu and Melissa Escobio are sisters and best friends who do just about everything together, including having retinitis pigmentosa (RP).
-

Dec 12, 2022
Resourceful Advocate for Research
Beacon StoriesManorthia was diagnosed with retinitis pigmentosa (RP) when she was 22 years old but didn’t start experiencing considerable vision loss until years later. Once she began to notice her vision deteriorating more rapidly, she reached out to the Foundation Fighting Blindness to get involved with the blindness community.
-

Oct 24, 2022
Charlie Kramer: Life Coach for the Disabled
Beacon StoriesRetinitis pigmentosa (RP) runs in Charlie Kramer’s family, so it wasn’t a surprise when he was diagnosed at a young age. But now, at 29 years old, Charlie is following his passion for helping and empowering others as a full-time life coach for those with disabilities.
-

Aug 15, 2022
Decades of Dedication Fighting for His Family and Himself
Beacon StoriesDan Day is one of over ten people in his family, spanning six generations, affected with retinitis pigmentosa (RP). So to “get off the sidelines and join the fight” for himself and his family, Dan has dedicated over 25 years of service to the Foundation Fighting Blindness.
-

Jun 6, 2022
Passionate Professional Outreach Volunteer Helping Newly Diagnosed
Beacon StoriesJim has always been avid about giving back to others, even after he was diagnosed with retinitis pigmentosa. And in the last few years, he’s begun working with the Foundation to help eye care professionals in the Cincinnati and Northern Kentucky area provide vital resources for their patients with retinal diseases.
-

May 23, 2022
A New Vision Through Music
Beacon StoriesMark Erelli has been a professional musician for the past twenty-three years. He’s always used songwriting to express himself. Since being diagnosed with retinitis pigmentosa, he’s needed music more than ever.
-

May 9, 2022
Cleanlogic Co-Founder’s Inspiration Comes From His Mom
Beacon StoriesIn honor of Mother’s Day, we’re sharing a special Beacon Story featuring Isaac Shapiro, co-founder of Cleanlogic. Isaac’s mother, Bea, lost her vision at only seven years old but went on to become an entrepreneur and help others in the blind and visually impaired community through adaptive technology.
-

Mar 28, 2022
Seeing Through Disability
Beacon StoriesIn her own words, Bari shares her perspective with having a father and brother affected with retinitis pigmentosa and how she’s now helping to make a difference to end blinding diseases.
-

Oct 25, 2021
Seeing Research Advances Firsthand
Beacon StoriesMark does not let his retinitis pigmentosa diagnosis keep him from doing what he loves most, including spending quality time with his family. And in 2019, Mark began participating in a clinical trial, which he describes as life-changing.
-

Oct 15, 2021
Learning to Fall After Vision Loss
Beacon StoriesWhen Justin’s vision loss progressed quickly due to retinitis pigmentosa at 25 years old, he thought skateboarding wouldn’t be possible anymore. Ten years later, with the help of his white cane and audio devices, Justin is now working to enhance accessibility in skateboarding for the blind and visually impaired community.
-

Sep 13, 2021
Telling His Story of Resilience
Beacon StoriesChad has been working in the corporate world for the last 20 years. But after becoming the first blind executive to graduate from the Harvard Business School leadership program, he realized his purpose in life is to help others by sharing the lessons he learned while losing his eyesight. He can now add published author to his repertoire with his new book, Blind Ambition.
-

Jul 26, 2021
Seeing Through Your Disability
Beacon Stories30-year-old Lance Johnson is a video editor and podcaster living in Brooklyn, New York. Despite being diagnosed with retinitis pigmentosa (RP) at a young age, Lance has never let his RP change his drive and passion for creating.
-
Jul 8, 2021
Fighting RP on the Foundation’s Frontline
Beacon StoriesMichelle Glaze, the Foundation’s associate director of professional outreach, shared her personal story of being diagnosed with retinitis pigmentosa (RP) in the film ‘Decoding disease.‘ In her own words, Michelle also describes her journey with genetic testing and the Foundation Fighting Blindness’ impact on her life.
-

Jun 7, 2021
Birding By Ear
Beacon StoriesFrom stargazing to bird watching, Michael has been an enthusiast of all things science from the age of 12. When Michael was diagnosed with retinitis pigmentosa at 30 years old, he knew he wasn’t going to give up his dream of becoming a record-setting birder. In his own words, Michael shares how he’s overcome his vision loss and continued to pursue his birding passions with “birding-by-ear.”
-

Apr 14, 2021
Audio Interview of Foundation Fighting Blindness Board Director Karen Petrou on Her New Book and Living with RP
Beacon StoriesAmerican Banker dubbed her as “the sharpest mind analyzing banking policy today – maybe ever.”
-

Mar 22, 2021
A Family Full of Hope
Beacon Stories18-year-old Marty Dubecky has a very unique decision to make. There are currently four gene therapy clinical trials for him to choose from to enroll in, which would have been unheard of 15 years ago when he was first diagnosed with XLRP.
-

Mar 8, 2021
George Dolan: Cherished Friend and Community Advocate
Beacon StoriesGeorge was well-known in his community for his integrity and dedication to helping everyone around him.
-
Feb 22, 2021
Managing Your Mindset, Embracing Your Voice
Beacon StoriesCharity’s recent rapid decline in vision due to RP and her daughter’s diagnosis with RP has caused her to open up about her vision loss journey. After getting involved with the Foundation, Charity now feels like she has a voice to help others in the visually impaired community.
-
.jpg)
Feb 8, 2021
Erin’s Unwavering Determination, Then and Now
Beacon StoriesDiagnosed with retinitis pigmentosa at a very young age, growing up, Erin never let her progressive vision loss stop her from keeping up with her friends and siblings. Throughout the 1990s, Erin was featured in several Foundation Fighting Blindness campaigns to raise funds for retinal research. Today, 34-year-old Erin is resilient as ever, still hopeful research will one day find her a cure.
-

Jan 25, 2021
Looking Past RP with Hope
Beacon StoriesJenny was diagnosed with retinitis pigmentosa (RP), just like her mom, at the age of 34. In her own words, Jenny shares her experience with being diagnosed with RP and her journey to accepting it with hopefulness.
-

Sep 25, 2020
ProQR Announces Virtual Presentations at Scientific Conferences
ResourceProQR Therapeutics N.V. (Nasdaq:PRQR), a company dedicated to changing lives through the creation of transformative RNA therapies for severe genetic rare diseases, today announced virtual presentations at the Ophthalmology Futures Retina Forum, European Society of Retina Specialists (Euretina) congress and the Annual Meeting of the American Academy of Optometry (AAOpt).
-

May 18, 2020
An Artist, First and Foremost
Beacon StoriesAllen has always wanted to be known as an artist, first and foremost. His photography hints at the ever-changing nature of people’s lives and their environment, much like his own progression with retinitis pigmentosa (RP).
-

Mar 9, 2020
No Slowing Down for LCA
Beacon StoriesBraydon was diagnosed with an inherited retinal disease at only two years old. Eight years later, after his mom enrolled him in the My Retina Tracker® Program, Braydon learned his disease was LCA.
-

Oct 15, 2019
Rachel Wants to Raise White Cane Awareness
Beacon StoriesRachel Luehrs describes her journey of acceptance.
-

Oct 11, 2019
The Bergstein’s Are Striking Out Blindness
DIY Campaign Success StoriesThe Bergstein family has always been passionate about helping the Foundation Fighting Blindness.
-

Sep 6, 2019
How Davida Regained Her Speed with Guide Dog Chubb
Beacon StoriesIn honor of National Guide Dog Month this September, Davida is sharing her experience with the Guide Dog Foundation for the Blind and how she found a perfect match in her new guide dog, Chubb.
-

Aug 5, 2019
Hannah Dreams Big Despite Vision Loss
Beacon StoriesHannah has always had dreams of starting her own fashion line. And despite being diagnosed with retinitis pigmentosa at the age of 15, she recently began an intimate company, Watson & Wilma.
-

Jul 10, 2019
My Retina Tracker, My Story
Beacon StoriesSusan, who has retinitis pigmentosa, went through many tests in search of learning more about her eye disease. Once she enrolled in the My Retina Tracker® (MRT) testing program, Susan was provided with comprehensive results and a clear diagnosis, giving her clarity and hope.
-

Jun 17, 2019
Jack Sees a Different Life after LUXTURNA
Beacon StoriesJack Hogan was diagnosed with retinitis pigmentosa at only two-and-a-half years old. But with the help of connections made with the Foundation, Jack became the first-ever recipient of the FDA-approved gene therapy known as LUXTURNA.
-

Jun 3, 2019
Original Foundation Advocate Gertrude Weiss Celebrates 100th Birthday
Beacon StoriesGertrude Weiss was one of the early Foundation Fighting Blindness investors and advocates. Diagnosed with retinitis pigmentosa, Gertrude continues to live her life to the fullest. As a longtime friend, the Foundation honored Gertrude on her 100th birthday recently.
-

Apr 26, 2019
Legally Blind Artist Paul Castle Expresses His Story through Art
Beacon StoriesPaul Castle was diagnosed with X-linked retinitis pigmentosa at the age of 16, but continued to follow his passion for art. Now Paul is a full-time artist and donates 5 percent of his art sales to the Foundation.
-

Jul 27, 2018
Persevering to Success with the Support of Family, Friends, and Faith
Beacon StoriesA story about living with retinitis pigmentosa.
-

Dec 17, 2017
Faith, Hope, and a Found Gene
Beacon Stories“I try not to get my hopes up too much, but I never lose hope; I’m determined. I just want to see my son’s face, see him get married, & see my grandchildren. I encourage everyone with a retinal disease to get a genetic test & to never ever give up hope.“
-

Apr 15, 2015
A Renaissance Man with Vision
Beacon StoriesAn interview with Louis Posen, record label founder and retinitis pigmentosa patient.
-

Jun 26, 2014
Lighting a Candle: The Author of a New Memoir Shares the Highs and Lows of Vision Loss
Beacon StoriesNicole Simpson, author of “Now I See You,” shares her experience with retinitis pigmentosa.
-

Sep 6, 2013
Alice Bartlett – Back on That Horse
Beacon StoriesHis name is Battle. He’s an Arabian, stands more than 15 hands (or 5 feet) tall and weighs 1,000 pounds. He’s been with Alice Bartlett since his birth 26 years ago, and she’s been riding him since he was 2.
Alice, by the way, is legally blind.
-

-

Mar 22, 2013
What Losing Vision Has Taught Me
Beacon StoriesAuthor Shawn Maloney describes his journey with RP.
-

Apr 12, 2012
Making Memories
Beacon StoriesThe parents of young children affected by retinal diseases walk a fine line – between making the best of a trying situation and preparing for the worst.
-

Mar 19, 2012
His Mother's Son
Beacon StoriesWhen it comes to describing Eric Fulton, “gregarious” is a more-than-appropriate adjective for the husband and father of two who, fittingly, is also communications manager at the Bethesda-based Clark Construction Group. “It comes naturally to me,” he says of his ability to mix it up with people. “The cause makes it easier. But, otherwise, I’m pretty outgoing; I like to talk to people. It’s what I do.”




